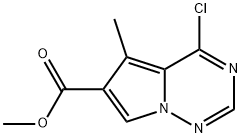
4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester synthesis
- Product Name:4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester
- CAS Number:310442-40-1
- Molecular formula:C9H8ClN3O2
- Molecular Weight:225.63
![Methyl 5-methyl-4-oxo-1,4-dihydropyrrolo[2,1-f][1,2,4]triazine-6-carboxylate](/CAS/GIF/310431-29-9.gif)
310431-29-9
46 suppliers
$45.00/10mg
![4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester](/CAS/GIF/310442-40-1.gif)
310442-40-1
22 suppliers
$504.32/5MG
Yield:310442-40-1 93%
Reaction Conditions:
with trichlorophosphate at 100; for 15 h;Product distribution / selectivity;
Steps:
27; 33 4-chloro-5-methyl-6-carbomethoxypyrrolo[2,1-f][1,2,4]triazine
Example 27 4-chloro-5-methyl-6-carbomethoxypyrrolo[2,1-f][1,2,4]triazine Phosphorous oxychloride (2.5 mL) was combined with Compound C of Example 19 (100 mg, 0.483 mmol) and heated at 100° C. overnight. The melt was allowed to cool to rt and dissolved in ethyl acetate. The mixture was neutralized with aqueous NaHCO3 and extracted twice with ethyl acetate. The combined organic washes were dried (Na2SO4) and concentrated to provide 101 mg (93%) of Example 27. MS: (M+H)+226.6 Example 33 5-Methyl-4-[[3-[(methylsulfonyl)amino]phenyl]amino]pyrrolo[2,1-f][1,2,4]triazine-6-carboxylic Acid Methyl EsterPhosphorus oxychloride was combined with Example 19 (30 mg, 0.14 mmol) and heated to 100° C. for 15 hrs. The melt was cooled and concentrated to remove excess reagent, then extracted with ethyl acetate (20 mL). The extract was washed with sat aqueous NaHCO3, (10 mL) dried over MgSO4 and concentrated in vacuo to provide chlorinated compound Example 27. The residue was dissolved in DMF (2 mL) and 3(methylsulfonylamino)aniline (54 mg, 0.3 mmol) was added. The reaction mixture was stirred for 4 hrs under argon at 25° C. The crude reaction mixture was purified by preparative HPLC. The material obtained appeared to be a salt of the desired compound. The material was dissolved in ethyl acetate and washed with saturated aqueous NaHCO3. Evaporation of the solvent gave 24 mg (40%) of Example 33. MS: [M+H]+=376.2; 1H NMR (d-DMSO): δ 8.89 (s, 1H), 8.14 (s, 1H), 7.95 (s, 1H), 7.55 (s, 1H), 7.38 (s, 1H), 7.34-7.32 (m, 2H), 7.01 (d, J=8.0 Hz, 1H), 3.81 (s, 3H), 3.02 (s, 3H), 2.82 (s, 3H).
References:
US6982265,2006,B1 Location in patent:Page/Page column 38; 41
40318-15-8
54 suppliers
$65.00/100mg
![4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester](/CAS/GIF/310442-40-1.gif)
310442-40-1
22 suppliers
$504.32/5MG
3780-42-5
25 suppliers
inquiry
![4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester](/CAS/GIF/310442-40-1.gif)
310442-40-1
22 suppliers
$504.32/5MG

623-43-8
190 suppliers
$11.00/10g
![4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester](/CAS/GIF/310442-40-1.gif)
310442-40-1
22 suppliers
$504.32/5MG
310431-26-6
16 suppliers
inquiry
![4-Chloro-5-methylpyrrolo[2,1-f][1,2,4]triazine-6-carboxylic acid methyl ester](/CAS/GIF/310442-40-1.gif)
310442-40-1
22 suppliers
$504.32/5MG