
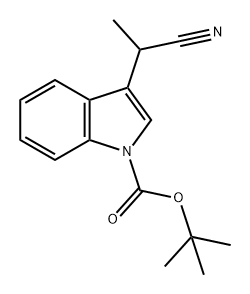
1H-Indole-1-carboxylic acid, 3-(1-cyano-1-methylethyl)-, 1,1-dimethylethyl ester synthesis
- Product Name:1H-Indole-1-carboxylic acid, 3-(1-cyano-1-methylethyl)-, 1,1-dimethylethyl ester
- CAS Number:380626-46-0
- Molecular formula:C17H20N2O2
- Molecular Weight:284.35

74-88-4
340 suppliers
$15.00/10g

380626-46-0
34 suppliers
inquiry
Yield:380626-46-0 71.7%
Reaction Conditions:
Stage #1: 3-(cyanomethyl)-1H-indol-1-carboxylic acid tert-butyl esterwith sodium hydroxide in N,N-dimethyl acetamide;water at 0; for 0.166667 h;
Stage #2: methyl iodide in N,N-dimethyl acetamide;water at 0 - 20; for 16 h;
Steps:
24; 25; 1.2
3-Cyanomcthyl-indolc-l-carboxylic acid tert-butyl ester (65) (40.0 g, 1.0 cq.) dissolved in N,N-dimethylacetamide (400 mL) was cooled in ice-water bath at 00C, then NaOH (18.728 g, 3.0 eq.) dissolved in H2O (18.728 mL) was added dropwise to the solution mixture. After stirring for 10 minutes, McI (66.46 g, 3.0 cq.) was slowly added while the reaction mixture was continued to cool at 00C. After addition, the mixture was slowly warmed up to room temperature and stirred overnight (16 hours). Precipitate was observed and the reaction was considered complete by LC/MS and TLC (Ry = 0.37; 10:90 EtOAc/Hex). Water (250 mL) was added to the reaction mixture and the precipitate was collected by filtration, and then rinsed with H2O several times to remove residual NaOH and DMA. More precipitate was formed in the filtrate, and it was collected by filtration once again as mentioned above. The precipitate was then rinsed with small amount of hexanes, dried overnight under vacuum to yield 3-(cyano-dimethyl-methyl)-indole-l-carboxylic acid tert-butyl ester (66) as an off-white solid (31.8 g 71.7%). 1H-NMR (400MHz, CDCl3): 8.18- 8.16 (br d, IH), 7.83-7.81 (dd, IH), 7.53 (s, IH), 7.39-7.28 (m, 2H), 1.85 (s, 6H), 1.68 (s, 9H).
References:
WO2007/70796,2007,A1 Location in patent:Page/Page column 110; 111; 117
![[1-(tert-Butoxycarbonyl)indol-3-yl]acetonitrile](/CAS/GIF/218772-62-4.gif)
218772-62-4
35 suppliers
inquiry

74-88-4
340 suppliers
$15.00/10g

380626-46-0
34 suppliers
inquiry
629662-14-2
0 suppliers
inquiry