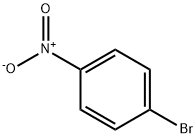
4-(2,2,2-TRIFLUOROETHOXY)NITROBENZENE synthesis
- Product Name:4-(2,2,2-TRIFLUOROETHOXY)NITROBENZENE
- CAS Number:62149-35-3
- Molecular formula:C8H6F3NO3
- Molecular Weight:221.13
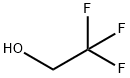
75-89-8
462 suppliers
$9.00/1g
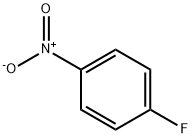
350-46-9
516 suppliers
$10.60/1gm:

62149-35-3
26 suppliers
$60.00/50mg
Yield:62149-35-3 98%
Reaction Conditions:
Stage #1: 2,2,2-trifluoroethanolwith sodium hydride in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: 4-Fluoronitrobenzene in tetrahydrofuran at 20; for 3 h;
Steps:
68 4-(2,2,2-Trifluoroethoxy)nitrobenzene
To a solution of 2,2,2-trifluoroethanol (26.49 g, 0.26 mol) in THF (300 mL) was added sodium hydride (in oil, 65%, 10.27 g, 0.28 mol) under ice-cooling, and the mixture was stirred for 1 hr at room temperature. To this mixture was added p-fluoronitrobenzene (37.36 g, 0.26 mol), and the mixture was stirred for 3 hr at room temperature. To the reaction mixture was added saturated aqueous sodium hydrogencarbonate, and the mixture was extracted twice with ethyl acetate. The organic layers were combined, washed with saturated brine, dried over magnesium sulfate and concentrated under reduced pressure to give the title compound (56.13 g, yield 98%) as a yellow amorphous solid. 1H-NMR (300MHz, CDCl3) δ:4.46 (2H, q, J = 7.8 Hz), 7.04 (2H, d, J = 9.2 Hz), 8.25(2H, d, J = 9.2 Hz).
References:
EP1810677,2007,A1 Location in patent:Page/Page column 61
100-02-7
5 suppliers
$11.00/5G
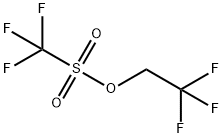
6226-25-1
254 suppliers
$11.19/5G

62149-35-3
26 suppliers
$60.00/50mg

75-89-8
462 suppliers
$9.00/1g
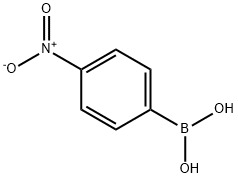
24067-17-2
251 suppliers
$19.00/1g

62149-35-3
26 suppliers
$60.00/50mg