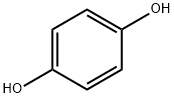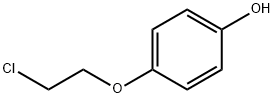
4-(2-CHLOROETHOXY)PHENOL synthesis
- Product Name:4-(2-CHLOROETHOXY)PHENOL
- CAS Number:100238-55-9
- Molecular formula:C8H9ClO2
- Molecular Weight:172.61
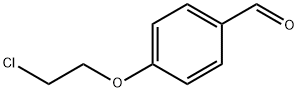
54373-15-8
71 suppliers
$68.00/5g

100238-55-9
17 suppliers
inquiry
Yield: 85%
Reaction Conditions:
with sulfuric acid;dihydrogen peroxide in methanol;water at 20; for 44 h;Cooling with ice;
Steps:
Synthesis of 4-(2-chloroethoxy)phenol (1a) by oxidation of 4-(2-chloroethoxy)benzaldehyde (4a) (Scheme 1, route B).
To a stirred solution of 4a (45.213 g, 0.245 mol) in MeOH (270 mL), 37% H2O2 (25.5 mL, 0.318 mol) was added and the resulting mixture was cooled in an ice-water bath (the aldehyde partially crystallized). Concentrated (95%) H2SO4 (2.75 mL, 0.049 mol) was added dropwise with stirring (the aldehyde dissolved and a fine crystalline precipitate of the peroxide intermediate was formed) and the reaction mixture was stirred for 44 h at room temperature (water bath was used to compensate for the slight exothermic effect) until TLC monitoring (PhMe-AcOEt (9 : 1), negative reaction with 2,4-dinitrophenylhydrazine) showed complete conversion of the starting aldehyde 4a (the precipitate dissolved within ∼16 h). The excess peroxide was destroyed by addition of 10% aqueous Na2SO3 (100 mL) with stirring (after an exothermic reaction ceased, the starch-iodine test was negative) and the reaction mixture was concentrated under reduced pressure. An aqueous solution containing 5% KOH and 2% Na2SO3 (350 mL) was added to the residue, followed by addition of ButOMe (100 mL). After shaking, the organic layer was separated and extracted with 5% aqueous KOH (3×50 mL). The dissolved ButOMe was removed from the combined alkaline aqueous extracts on a rotary evaporator (until the beginning of distillation of water) and solid CO2 (100 g) was slowly added to the resulting solution. The precipitate that formed was collected by suction filtration, washed with ice cold water (3×150 mL), and dried in a vacuum desiccator over solid KOH to give 34.988 g of compound 1a (as colorless crystals) identical to that described above. Additional crop (0.863 g) of 1a was recovered from the mother liquor by extraction with ButOMe, reextraction with 5% aqueous KOH, and addition of solid CO2 to the aqueous phase. Overall yield of 1a was 35.851 g (85%).
References:
Zinin;Stepanova;Jost;Kondakov;Shpirt;Chizhov;Torgov;Kononov [Russian Chemical Bulletin,2017,vol. 66,# 2,p. 304 - 312][Izv. Akad. Nauk, Ser. Khim.,2017,# 2,p. 304 - 312,9]
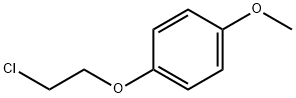
3383-74-2
12 suppliers
$45.00/100mg

100238-55-9
17 suppliers
inquiry