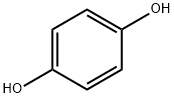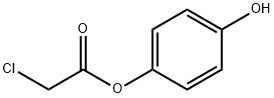
p-Hydroxyphenyl chloroacetate synthesis
- Product Name:p-Hydroxyphenyl chloroacetate
- CAS Number:10421-12-2
- Molecular formula:C8H7ClO3
- Molecular Weight:186.59
Yield:10421-12-2 85%
Reaction Conditions:
in N,N-dimethyl-formamide; for 0.75 h;Inert atmosphere;
Steps:
3.2.1. Synthesis of 4-Hydroxyphenyl 2-chloroacetate (1)
Hydroquinone (1.00 g, 9.08 mmol) was dissolved in DMF (18.1 mL). Chloroacetyl chloride(0.866 mL, 10.9 mmol) was added dropwise under a positive nitrogen atmosphere over 10 min and theclear solution soon became pale yellow. After 45 min the reaction was quenched by addition of 20 mLH2O and the white precipitated diester was filtered o. The filtrate was extracted with Et2O (20 mL 3).The combined organic layers were washed with H2O (30 mL 2) and brine, dried over MgSO4 andconcentrated in vacuo to yield the crude compound 1 as a white powder. The crude was recrystallizedin H2O and pure compound 1 was obtained as white crystals (1.44 g, 7.72 mmol, 85%). 1H-NMR(500 MHz, CDCl3): δ 6.99 (d, Har, 2H), 6.84 (d, Har, 2H), 4.28 (s, Cl-CH2-CO, 2H) 13C-NMR (300 MHz,CDCl3): δ 153.75 (C4) 122.29 (Car), 116.21 (Car), 41.01 (Cl-C-CO) ESI+-MS: C8H7ClO3 [M + Na]+ m/zcalc208.99759 m/zfound 208.99778 abs (HEPES 0.1 M, pH 7.2) = 275 nm, 221 nm.
References:
Del Giorgio, Elena;S?rensen, Thomas Just [Molecules,2020,vol. 25,# 8]