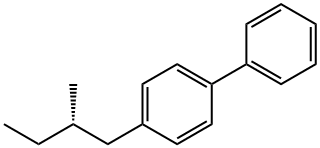
4-[(2S)-2-Methylbutyl]-1,1'-biphenyl synthesis
- Product Name:4-[(2S)-2-Methylbutyl]-1,1'-biphenyl
- CAS Number:62614-28-2
- Molecular formula:C17H20
- Molecular Weight:224.34
Yield:62614-28-2 66%
Reaction Conditions:
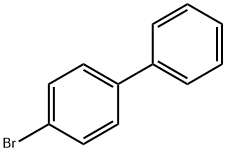
Stage #1: 4-bromo-1,1'-biphenylwith magnesium in tetrahydrofuran; for 1 h;Inert atmosphere;Reflux;
Stage #2: (S)-2-methylbutyl bromidewith dilithium tetrachlorocuprate in tetrahydrofuran; for 48 h;Reflux;Inert atmosphere;
Steps:
Coupling of alkyl bromides with aryl Grignards -general procedure. Synthesis of (S)-1-fluoro-3-(2-methyl-1-butyl)benzene (3):
A solution of (262.5g , 1.5 mol) 1-bromo-3-fluorobenzenein anhydrous THF (1 L) was added dropwise to Mg chips (36.5g,1.5mol) with vigorous stirring under N2 atmosphere. The mixture wasrefluxed for 1 h, then a 1 mol/dm3 solution of catalyst Li2CuCl4in anhydrous THF (45 mL, 3mol%) was added. Next, (S)-1-bromo-2-methylbutane(214.2g , 1.58 mol) was added dropwise to the mixture which wasstirred under reflux for 2 days. When the reaction was completed the mixturewas poured into 1500 mL of H2O and 100 ml of 10% HCl. The crudeproduct was extracted with pentane. The organic layer was washed with H2O(2 x 300 mL), separated, dried over MgSO4 and the solventevaporated. The product was distilled under reduced pressure: b.p. 98-100 oC(28 mmHg), yield 205 g (83%), [α]D25= 9.5° (c=5.1; CHCl3).MS(EI) m/z: 166 (M+), 133,110, 96.1H NMR (CDCl3), δ 7.65-7.60(m, 1H), 7.53 (t, J = 2.2, 3.1Hz,1H), 6.82-6.75 (m, 2H), 2.50 (d, J =8.8 Hz, 2H), 1.60-1.58 (m, 1H), 1.35-1.25 (m, 2H), 0.93 (t, J = 12.2 Hz, 3H), 0.86 (t, J = 11.4 Hz, 3H). 13C NMR (CDCl3) δ11.65, 19.0, 29.4,36.6, 42.3, 109.0, 115.5, 121.7, 124.9, 126.4, 128.9, 145.0, 161.4.
References:
Herman, Jakub;Kula, Przemys?aw [Tetrahedron Letters,2013,vol. 54,# 28,p. 3621 - 3623]

93560-34-0
0 suppliers
inquiry
![4-[(2S)-2-Methylbutyl]-1,1'-biphenyl](/CAS2/GIF/62614-28-2.gif)
62614-28-2
9 suppliers
inquiry

534-00-9
55 suppliers
$70.00/100mg
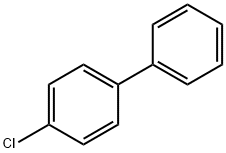
2051-62-9
133 suppliers
$35.00/250mg
![4-[(2S)-2-Methylbutyl]-1,1'-biphenyl](/CAS2/GIF/62614-28-2.gif)
62614-28-2
9 suppliers
inquiry