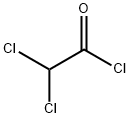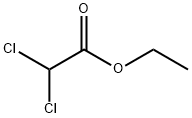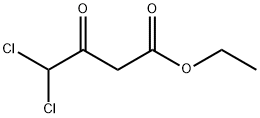
4,4-dichloro-3-oxobutyric acid ethyl ester synthesis
- Product Name:4,4-dichloro-3-oxobutyric acid ethyl ester
- CAS Number:6082-74-2
- Molecular formula:C6H8Cl2O3
- Molecular Weight:199.03
Yield:6082-74-2 85.7%
Reaction Conditions:
Stage #1: ethyl potassium malonatewith triethylamine;magnesium chloride in acetonitrile at 10 - 25;
Stage #2: dichloroacethyl chloride in acetonitrile at 0 - 25; for 18 h;
Steps:
5 Example 5
Maintain the internal temperature of 10 ~ 25 ,100 g of the potassium salt of monoethyl malonate obtained in Example 1 was obtained(HPLC purity: 98.8%, 0.581 mol) and 700 g of acetonitrile were mixed in a reaction flask to maintain the internal temperature of the system at 10 ° C to 25 ° C. After adding 96.23 g of triethylamine (0.951 mol)A solution of 66.35 g of magnesium chloride solids (0.696 mol) was slowly added. After the addition is complete,Natural temperature, and keep the system temperature 20 ~ 25 insulation 2 to 3 hours.After cooling to 0 to 10 ° C, 64.24 g of dichloroacetyl chloride (0.436 mol) was slowly added dropwise. Drop finished,The temperature was naturally raised to 20 ° C to 25 ° C and held at that temperature for 18 hours.After completion of the reaction, 220 g of ethyl acetate was added to dilute the reaction solution. Cooling to 0 ~ 15 , slowly dropping 420gOf the aqueous solution of hydrochloric acid having a mass concentration of 5% (the mass concentration means that the mass of the hydrogen chloride accounts for the total amount of the aqueous hydrochloric acid solutionPercentage of mass). After completion of the dropwise addition, the mixture was incubated at 0 to 15 ° C for 30 minutes. The resulting organic phase uses a mass concentrationAnd washed twice with 5% aqueous hydrochloric acid (180 g x 2). After the obtained organic phase was removed from the solvent,Ethyl dichloroacetoacetate76.12 g (GC purity: 97.7%, yield: 85.7%).
References:
CN106467492,2017,A Location in patent:Paragraph 0137; 0138; 0139
105-53-3
735 suppliers
$5.00/25g

6082-74-2
10 suppliers
inquiry