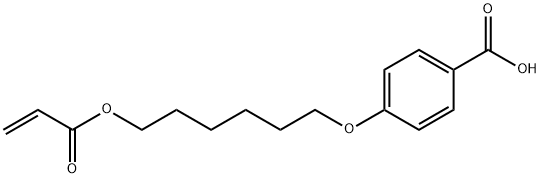
4-((6-(Acryloyloxy)hexyl)oxy)benzoic acid synthesis
- Product Name:4-((6-(Acryloyloxy)hexyl)oxy)benzoic acid
- CAS Number:83883-26-5
- Molecular formula:C16H20O5
- Molecular Weight:292.33
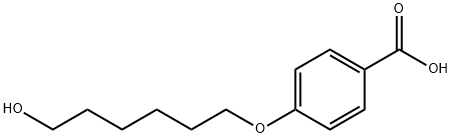
83883-25-4
46 suppliers
$79.00/1G

814-68-6
381 suppliers
$21.21/5gm:

83883-26-5
140 suppliers
$8.00/250mg
Yield:83883-26-5 100%
Reaction Conditions:
in ISOPROPYLAMIDE at 25 - 30; for 1.25 h;Product distribution / selectivity;
Steps:
E.3
Example 3 (E 3) ; synthesis of compound 1:; In a 250 ml chemical reactor are dissolved 23.89 G (0.10 mol) of 4- (6-hydroxyhexyloxy) benzoic acid (PURITY-100%, preparation e. g. literature mentioned in example 1) in 100 G of dimethyl- acetamide at ca. 20°C. To the dear, stirred solution are gradually added 10.26 g (0.11 mol) of acryloyl chloride (Aldrich 96%), keeping the reaction temperature below 35°C by external cooling. After the addition, which takes about 15 minutes, the reaction mixture is stirred for 1 hours at 30°C, when TLC analysis (Silicagel, Cyclohexane : ETHYLACETATE : Acetic acid = 50: 50: 2, parts b. volume, UV fluorescence indicator) indicates completion of the reaction. A nearly colourless suspension is formed. It is gradually precipitated into 400 ml of cold water using a high speed stirrer (1300 rpm). After finishing the addition, the suspension is stirred for ?hour and filtered. The filter residue is washed twice with warm (-40°C) water (400 ml), filtered and the residue dried in vacuo at 25°C. The thus obtained colourless, crystalline solid is dissolved in 93.40 G toluene at 70°C, 0.14 9 of di-tert. butyl-p-cresole (BHT, polymerisation inhibitor) added and the solution cooled slowly to 0°C. The crystalline precipitate is filtered off and dried in vacuum at 35°C to constant weight. 24.40 G (83.5% o. th. ) of colourless, crystalline 4- (6-ACRYLOYLOXYHEXYLOXY) benzoic acid = compound (1) are obtained.
References:
WO2005/30696,2005,A1 Location in patent:Page/Page column 11-12

83883-25-4
46 suppliers
$79.00/1G
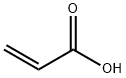
79-10-7
688 suppliers
$20.50/500ml

83883-26-5
140 suppliers
$8.00/250mg

4286-55-9
298 suppliers
$15.00/10g

83883-26-5
140 suppliers
$8.00/250mg
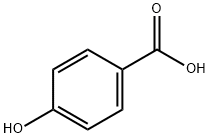
99-96-7
770 suppliers
$5.00/10g

83883-26-5
140 suppliers
$8.00/250mg

2009-83-8
361 suppliers
$7.00/25g

83883-26-5
140 suppliers
$8.00/250mg