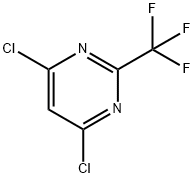
4,6-dichloro-2-(trifluoromethyl)pyrimidine synthesis
- Product Name:4,6-dichloro-2-(trifluoromethyl)pyrimidine
- CAS Number:705-24-8
- Molecular formula:C5HCl2F3N2
- Molecular Weight:216.98
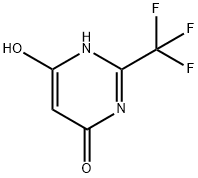
672-47-9
71 suppliers
$45.00/50mg

705-24-8
108 suppliers
$26.60/250mg
Yield: 78.8%
Reaction Conditions:
with trichlorophosphate in water;triethylamine
Steps:
2 4,6-Dichloro-2-trifluoromethylpyrimidine, Compound V, Step 2
EXAMPLE 2 4,6-Dichloro-2-trifluoromethylpyrimidine, Compound V, Step 2 4,6-Dihydroxy-2-trifluoromethylpyrimidine (VI; 540 g; 3.0 M) was stirred with phosphorous oxychloride (1300 mL; 2147 g; 14.0 M) in a 5 L round-bottom flask with mechanical stirrer and triethylamine (607 g; 6.0 M) was added over 1 hr. After the exotherm, the reaction was heated on a stem-bath for 3 hr. The reaction mixture was cooled to 40°, and then it was poured with stirring into a mixture of 10 kg ice and 2 kg water. The mixture was extracted with 4*0.5 L methylene chloride. The combined extracts were dried (MgSO4) and filtered. The filtrate was concentrated in vacuo to give the crude compound V. When further purification was desired, the crude V was distilled at 48°-50°/2 mm using a steam-bath (Lit. b.p. 38°/1 mm) to give a colorless liquid. The purified yield was 78.8%. Anal. Calcd. for C5 HCl2 F3 N2: C, 27.68; H, 0.47; N, 12.91. Found: C, 27.45; H, 0.39; N, 12.86.
References:
Bristol-Myers Squibb Co. US4963678, 1990, A