
4'-BENZYLOXY-BIPHENYL-3-YLAMINE synthesis
- Product Name:4'-BENZYLOXY-BIPHENYL-3-YLAMINE
- CAS Number:400744-34-5
- Molecular formula:C19H17NO
- Molecular Weight:275.34
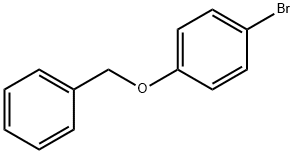
6793-92-6
307 suppliers
$6.00/5g
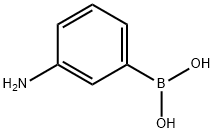
30418-59-8
248 suppliers
$6.00/1g

400744-34-5
26 suppliers
$531.00/5g
Yield:400744-34-5 63%
Reaction Conditions:
with tetrakis-(triphenylphosphine)-palladium;potassium carbonate in 1,4-dioxane;water monomer; for 5 h;Inert atmosphere;Reflux;Suzuki Coupling;
Steps:
4'-(Benzyloxy)-[1,1'-biphenyl]-3-amine(19).
K2CO3(3.35g,24.3 mmol) was added to a solution of 4-bromophenol 17(17.3 mmol, 3.00 g) in dry acetonitrile, and the mixture was thenstirred for 30 min. Benzyl bromide (17.3 mmol, 2.06 mL) was addedand the resulting mixture was stirred overnight. The mixture wasfiltered through Celite and the solvent was evaporated. Theresulting solid was triturated from hexane to give pure compound1-(benzyloxy)-4-bromobenzene 18 (yield 50%). Pd[(C6H5)3P]4(263 mg, 0.22 mmol, 0.20 equiv.) was added to a solution of 18(300 mg, 1.14 mmol, 1.00 eq) andK2CO3(945 mg, 6.84 mmol, 6.00eq) in a dioxane/water mixture (8:2 v/v). After the resultingmixture was stirred under a nitrogen atmosphere for 1 h at rt, (3-aminophenyl)boronic acid (2.28 mmol, 2.0 eq) was added. The reactionmixture was heated at reflux under a nitrogen atmospherefor 4 h, then it was cooled to room temperature, diluted with waterand the mixture was extracted three times with EtOAc. The combinedorganic layers were dried overNa2SO4and concentratedunder reduced pressure. The crude product was purified by flaschromatography (eluent: petroleum ether/EtOAc 8/2 v/v and thenDCM) to give aniline 19 as an oil (Yield 63%). 1HNMR (600 MHz,DMSO-d6):5.14 (s, 2H), 5.88 (very br s, 2H), 6.60 (d, J7.4 Hz, 1H,d6.83 (d, J7.3 Hz, 1H), 6.87 (s, 1H), 7.16e7.04 (m, 3H), 7.36e7.33 (m,1H), 7.41 (t, J7.5 Hz, 2H), 7.53e7.44 (m, 4H).
References:
Pippione, Agnese Chiara;Kilic-Kurt, Zühal;Kovachka, Sandra;Sainas, Stefano;Rolando, Barbara;Denasio, Enrica;Pors, Klaus;Adinolfi, Salvatore;Zonari, Daniele;Bagnati, Renzo;Lolli, Marco Lucio;Spyrakis, Francesca;Oliaro-Bosso, Simonetta;Boschi, Donatella [European Journal of Medicinal Chemistry,2022,vol. 237,art. no. 114366]
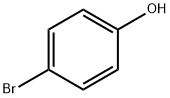
106-41-2
482 suppliers
$13.00/5g

400744-34-5
26 suppliers
$531.00/5g

100-39-0
425 suppliers
$10.00/10g

400744-34-5
26 suppliers
$531.00/5g