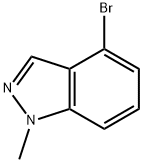
4-BROMO-1-METHYL-1H-INDAZOLE synthesis
- Product Name:4-BROMO-1-METHYL-1H-INDAZOLE
- CAS Number:365427-30-1
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06

74-88-4
340 suppliers
$15.00/10g
186407-74-9
310 suppliers
$5.00/1g

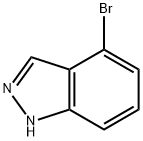
365427-30-1
96 suppliers
$42.00/1g
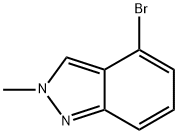
590417-93-9
122 suppliers
$45.00/250mg
Yield: 59.8% , 24.3%
Reaction Conditions:
Stage #1:4-bromoindazole with potassium carbonate in N,N-dimethyl-formamide for 0.5 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 25; for 3 h;
Steps:
5
K2CO3 (5.26 g, 38.06 mmol) was added to a mixture of 4-bromo-lHindazole(5 g, 25.38 mmol) in DMF (50 mL). 30 min later, Mel (18.2 g, 128.22 mmol, 8.0mL) was added and the mixture was stirred at 25 oc for 3h. The mixture was treated withH20 (150 mL) and EA (50 mL). The organic layer was separated and the aqueous layer wasextracted with EA (50 mL x 2). The combined organic layer was washed brine (50 mL x 2),dried over MgS04, filtered and concentrated. The residue was purified by flash columnchromatography over silica gel (PE/EA = 10/l to 5/1) to afford a pair of isomers. Isomer 1 (Compound 32A, Rf '" 0.54, PE/EA '" 5/1): 4-bromo-1-methylindazole(3.2 g, 59.8% yield) was obtained as white solid. 1H NMR (DMSO-d6, 400 MHz): ,57.98 (d, J '" 0.9 Hz, HI), 7.67- 7.65 (m, HI), 7.35- 7.27 (m, 2H), 4.04 (s, 3H). Isomer 2 (Compound 32B, Rr '" 0.24, PE/EA '" 5/1): 4-bromo-2-methylindazole(1.3 g, 24.3% yield) was obtained as colorless sticky oiL 1H NMR (DMSO-d6, 400MHz): J 8.37 (s, 1H), 7.60- 7.57 (m, 1H), 7.26- 7.21 (m, 1H), 7.13 (dd, Jooo7.3, 8.6 Hz, lH),4.16 (s, 3H).
References:
BLADE THERAPEUTICS, INC.;BUCKMAN, Brad Owen;YUAN, Shendong;EMAYAN, Kumaraswamy;ADLER, Marc;IBRAHIM, Prabha WO2019/190885, 2019, A1 Location in patent:Paragraph 0335-0337
360575-28-6
275 suppliers
$10.00/1g
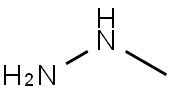
60-34-4
2 suppliers
$51.79/25g

365427-30-1
96 suppliers
$42.00/1g

74-88-4
340 suppliers
$15.00/10g

186407-74-9
310 suppliers
$5.00/1g

365427-30-1
96 suppliers
$42.00/1g
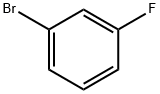
1073-06-9
428 suppliers
$6.00/10g

365427-30-1
96 suppliers
$42.00/1g
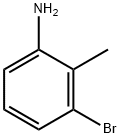
55289-36-6
279 suppliers
$5.00/1g

365427-30-1
96 suppliers
$42.00/1g

590417-93-9
122 suppliers
$45.00/250mg