
4-Chlorophenyl cyclopropyl ketone synthesis
- Product Name:4-Chlorophenyl cyclopropyl ketone
- CAS Number:6640-25-1
- Molecular formula:C10H9ClO
- Molecular Weight:180.63
873-77-8
147 suppliers
$61.21/100ml
5500-21-0
311 suppliers
$16.00/5mL

6640-25-1
163 suppliers
$21.00/1g
Yield:6640-25-1 705 mg
Reaction Conditions:
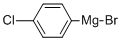
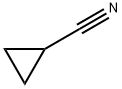
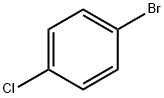
Stage #1: (4-chlorphenyl)magnesium bromide;cyclopropropanecarbonitrile in diethyl ether at 0 - 26; for 8 h;
Stage #2: with hydrogenchloride;water at 22 - 26;
Steps:
1 Step 1: (4-Chlorophenyl)(cyclopropyl)methanone
To a stirred solution of cyclopropylcarbonitrile (607 mg, 9.04 mmol) in anhydrous diethyl ether (25 mL) was slowly added 4-chlorophenylmagnessium bromide (1 , 11 mL, 11.76 mmol) at 0 °C. The reaction mixture was gradually warmed up to RT in duration of 2 h and continued to stir for another 6 h at RT. To that mixture were added IN HCl (11 mL) and THF (11 mL) and continued to stir overnight at RT. The reaction mixture was cooled to 0 °C and quenched with saturated aqueous solution of ammonium chloride (50 mL) and ethyl acetate (50 mL). The layers were separated and the aqueous layer was extracted with ethyl acetate (50 mL x 2). The combined organic layers were washed with brine (50 mL) and concentrated under reduced pressure. The residue obtained was purified by silica gel column chromatography to yield 705 mg of the titled product. 1H NMR (300 MHz, CDC13) δ 1.03- 1.09 (m, 2H), 1.22-1.28 (m, 2H), 2.57-2.67 (m, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.95 (d, J = 8.4 Hz, 2H).
References:
WO2017/37595,2017,A1 Location in patent:Page/Page column 27; 41
106-39-8
459 suppliers
$14.00/5g
147356-78-3
88 suppliers
$25.00/1g

6640-25-1
163 suppliers
$21.00/1g

18228-43-8
10 suppliers
$28.60/1g

6640-25-1
163 suppliers
$21.00/1g
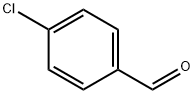
104-88-1
632 suppliers
$5.00/25g

6640-25-1
163 suppliers
$21.00/1g
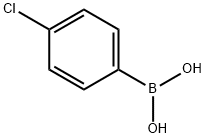
1679-18-1
610 suppliers
$10.00/1g

5500-21-0
311 suppliers
$16.00/5mL

6640-25-1
163 suppliers
$21.00/1g