
4-fluoro-2-Methoxy-5-nitroaniline synthesis
- Product Name:4-fluoro-2-Methoxy-5-nitroaniline
- CAS Number:1075705-01-9
- Molecular formula:C7H7FN2O3
- Molecular Weight:186.14
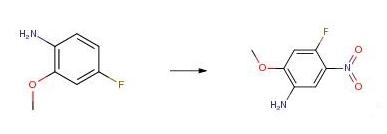
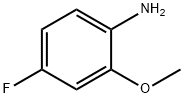
450-91-9
225 suppliers
$8.00/1g

1075705-01-9
340 suppliers
$8.00/1g
Yield:1075705-01-9 95%
Reaction Conditions:
with sulfuric acid;potassium nitrate at 0 - 5; for 2 h;Inert atmosphere;
Steps:
81 Preparation 81 : 4-fluoro-2-methoxy-5-nitroaniline
A solution of 4-fluoro-2-methoxyaniline (1590 mg, 11.27 mmol) in conc.H2S04 (9.55 ml_) was treated with solid KNO3 (1140 mg, 11.3 mmol) in portions while keeping the internal temperature below 5 °C. The resulting mixture was stirred for 2 h at 0 °C and mixture was poured into ice water (100 ml_), neutralized slowly with solid Na2CO3 and extracted with EtOAc (2 x 60 ml_). The organic layers were dried (Na2SO4), filtered and and concentrated to give the title compound (2 g, 95%).1H NMR (400 MHz, CDCl3) d 7.42 (d, 1 H), 6.66 (d, 1 H), 3.96 (s, 3H), 1.60 (s, 2H); LC/MS m/z (M+H)+=186.8.
References:
WO2021/124155,2021,A1 Location in patent:Page/Page column 53; 93
394-18-3
12 suppliers
inquiry

1075705-01-9
340 suppliers
$8.00/1g
448-19-1
296 suppliers
$5.00/1g

1075705-01-9
340 suppliers
$8.00/1g

124-41-4
686 suppliers
$12.00/25g

123344-02-5
85 suppliers
inquiry

1075705-01-9
340 suppliers
$8.00/1g
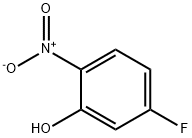
446-36-6
257 suppliers
$9.00/10g

1075705-01-9
340 suppliers
$8.00/1g