
4-HYDRAZINO-2-(TRIFLUOROMETHYL)QUINAZOLINE synthesis
- Product Name:4-HYDRAZINO-2-(TRIFLUOROMETHYL)QUINAZOLINE
- CAS Number:154136-31-9
- Molecular formula:C9H7F3N4
- Molecular Weight:228.17
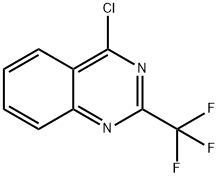
52353-35-2
65 suppliers
$85.00/250 mg

154136-31-9
23 suppliers
inquiry
Yield: 56%
Reaction Conditions:
with potassium carbonate;hydrazine in tetrahydrofuran at 20; for 2 h;
Steps:
3.1
Example 3; N-(l-/?-Tolyl-ethylidene)-N'-(2-trifluoromethyl-quinazolin-4-yl)-hvdrazine; Step 1; (2-Trifluoromethyl-quinazolin-4-yl)-hydrazine; To a solution of 4-chloro-2-(trifluoromethyl)quinazoline (2.0 g, 8.6 mmol) and hydrazine (0.27 mL, 8.6 mmol) in 20 mL of tetrahydrofuran was added 1.8 g (13 mmol) of potassium carbonate. The resultant suspension was stirred at ambient temperature for 2 hours. The reaction mixture was partitioned between ethyl acetate and water. The aqueous layer was extracted with ethyl acetate and the combined organics were dried over magnesium sulfate. After filtration, the organics were concentrated in vacuo to yield a yellow solid that was recrystallized in ethyl acetate: hexanes. 1.1 g (56%) of yellow needle-like crystals were isolated.LCMS: Mass Found (M+l, 229), 1H NMR: (OMSO-d6, 400 MHz) δ 10.2 (bs, IH), 8.28 (d, J = 8.06 Hz, IH), 7.81-7.89 (m, 2H), 4.96 (bs, 2H).
References:
APPLIED RESEARCH SYSTEMS ARS HOLDING N.V. WO2007/65940, 2007, A1 Location in patent:Page/Page column 35
![SODIUM 2-[(TRIFLUOROACETYL)AMINO]BENZOATE](/CAS/GIF/19165-29-8.gif)
19165-29-8
12 suppliers
$63.00/200 μmol

154136-31-9
23 suppliers
inquiry
16062-71-8
10 suppliers
$189.00/500mg

154136-31-9
23 suppliers
inquiry

26059-81-4
46 suppliers
$45.00/25mg

154136-31-9
23 suppliers
inquiry