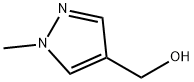
(1-Methyl-1H-pyrazol-4-yl)methanol synthesis
- Product Name:(1-Methyl-1H-pyrazol-4-yl)methanol
- CAS Number:112029-98-8
- Molecular formula:C5H8N2O
- Molecular Weight:112.13
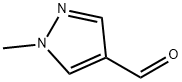
25016-11-9
239 suppliers
$5.00/1g

112029-98-8
108 suppliers
$18.00/1g
Yield: 97%
Reaction Conditions:
Stage #1:1-methylpyrazole-4-carbaldehyde with sodium tetrahydroborate in methanol at 20; for 3 h;
Stage #2: with hydrogenchloride;methanol in water; pH=1 at 0 - 20;
Steps:
3
The compound of formula IX (3.48 g, 31.6 mmol) was dissolved in methanol (25 ml_) and sodium borohydride (2.50 g, 66.1 mmol, 2.09 equiv.) was added portion-wise with vigorous gas evolution. After stirring for 3 hours at room temperature, the reaction was cooled to 0 0C and slowly acidified to pH ~ 1 with 4N aqueous hydrochloric acid (20 ml_) over 55 minutes. A thick white slurry formed and this was stirred one hour at room temperature. The reaction was then basified by the gradual addition of saturated aqueous potassium carbonate solution (53.4 wt% K2CU3, 6.04 M; 10 ml_). This resulted in a clear, colorless solution (pH = 1.1), which was diluted with additional saturated potassium carbonate solution (200 ml_) and was extracted with ethyl acetate (2 x 200 ml_). The ethyl acetate extracts were combined, dried over sodium sulfate, filtered and concentrated to yield the compound of formula X as a light yellow oil (3.44 g, 97% yield).
References:
SCHERING CORPORATION WO2008/153870, 2008, A1 Location in patent:Page/Page column 30
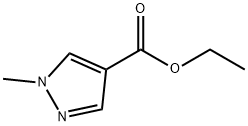
85290-80-8
93 suppliers
$44.00/250 mg

112029-98-8
108 suppliers
$18.00/1g

100852-80-0
57 suppliers
$57.50/250 mg

112029-98-8
108 suppliers
$18.00/1g