
4-methyl-3-Morpholinone synthesis
- Product Name:4-methyl-3-Morpholinone
- CAS Number:20721-78-2
- Molecular formula:C5H9NO2
- Molecular Weight:115.13

109-83-1
460 suppliers
$10.00/25ml

79-04-9
382 suppliers
$12.00/5g

20721-78-2
37 suppliers
$79.00/250mg
Yield:20721-78-2 58%
Reaction Conditions:
with sodium hydroxide in ethanol;lithium hydroxide monohydrate at 15 - 20; for 1.33333 h;
Steps:
65.A Step A: 4-methylmorpholin-3-one
A solution of 2-(methylamino)ethanol (5.32 mL, 66.6 mmol, 1 eq) in ethanol (100 mL) and 35% aqueous sodium hydroxide (6.25 mL) was cooled to 15-20 oC and chloroacetyl chloride (13.3 mL, 166 mmol, 2.5 eq) and 35% aqueous sodium hydroxide (22 mL) were added simultaneously with vigorous stirring over 1 h. The mixture was stirred for 20 min, then neutralised with aqueous hydrochloric acid and extracted with dichloromethane (3 x 100 mL). The combined organic extracts were washed with water, dried (PTFE phase separator) and concentrated in vacuo. Purification by automated flash column chromatography (CombiFlash Rf, 80 g RediSep silica cartridge) eluting with a gradient of 0- 100% ethyl acetate in iso-heptane afforded the desired product as a colourless oil (4.4 g, 38.2 mmol, 58%). 1H NMR (400 MHz, DMSO-d6) d 4.00 (s, 2H), 3.84- 3.78 (m, 2H), 3.36- 3.29 (m, 2H), 2.86 (s, 3H).
References:
WO2021/18858,2021,A1 Location in patent:Page/Page column 246
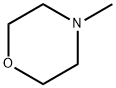
109-02-4
512 suppliers
$10.00/5ml

20721-78-2
37 suppliers
$79.00/250mg

109-02-4
512 suppliers
$10.00/5ml

18424-96-9
47 suppliers
$33.00/100mg

20721-78-2
37 suppliers
$79.00/250mg
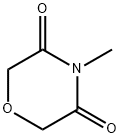
57503-67-0
3 suppliers
inquiry
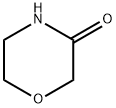
109-11-5
267 suppliers
$5.00/1g

74-88-4
357 suppliers
$15.00/10g

20721-78-2
37 suppliers
$79.00/250mg