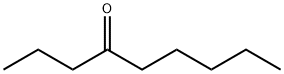
4-Methylenenonane synthesis
- Product Name:4-Methylenenonane
- CAS Number:33717-91-8
- Molecular formula:
- Molecular Weight:0

109-67-1
160 suppliers
$35.00/25g

33717-91-8
1 suppliers
inquiry
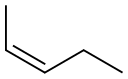
627-20-3
49 suppliers
$59.00/1g
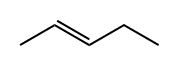
646-04-8
62 suppliers
$45.00/500mg

19150-21-1
18 suppliers
inquiry
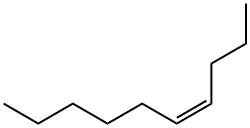
19398-88-0
11 suppliers
inquiry

19398-89-1
19 suppliers
inquiry

19398-86-8
7 suppliers
inquiry
Yield:33717-91-8 2.8%
Reaction Conditions:
2-[1-(2,4,6-trimethylphenylimino)ethyl]-6-[1-(4-eicosanoxy-3,5-diphenylphenylimino)ethyl]pyridine cobalt[II] chloride tetrakis[3,5-bis(trifluoromethyl)phenyl]borate;trimethylsilylmethyllithium at 20; under 750.075 Torr;Product distribution / selectivity;
Steps:
30
In an inert atmosphere (in dry box) in a stirred vessel at ambient temperature, 1-pentene(4. 6 g; 65. 7 mmol) was dimerised to a mixture of linear cis and trans 3-decene and 4-decene (2.8% wt 2-propyl-l-heptene is the main branched impurity), using 11.3 pmol cationic Co [II] catalyst,2- [l- (2, 4,6-trimethylphenylimino) ethyl]-6- [l- (4-eicosanoxy-3, 5-diphenylphenylimino) ethyl] pyridine cobalt [II] chloride tetrakis [3,5-
References:
WO2005/90371,2005,A1 Location in patent:Page/Page column 90-93

109-67-1
160 suppliers
$35.00/25g

33717-91-8
1 suppliers
inquiry
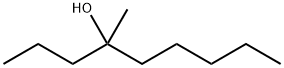
23418-38-4
32 suppliers
$45.00/50mg
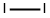
7553-56-2
617 suppliers
$19.00/25g

33717-91-8
1 suppliers
inquiry
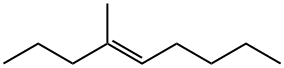
25147-82-4
0 suppliers
inquiry
29901-56-2
0 suppliers
inquiry