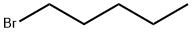4-N-PENTYLOXYBENZALDEHYDE synthesis
- Product Name:4-N-PENTYLOXYBENZALDEHYDE
- CAS Number:5736-91-4
- Molecular formula:C12H16O2
- Molecular Weight:192.25
Yield: 95.3%
Reaction Conditions:
with potassium carbonate in acetone at 60 - 70; for 24 h;Inert atmosphere;
Steps:
Preparation of 4-(pentyloxy)benzaldehyde (14)
To a suspension of 4-hydroxy benzaldehyde 13 (30.0 g, 0.245 mol) and powdered potassium carbonate (135.6 g, 0.982 mol) in acetone (600 mL, 20.0 Vol), 1-bromopentane (48.23 g, 0.319 mol) was slowly added at RT over the period of 20 min then heated to 60-70 °C and stirred for 24h. After completion of starting material, reaction mixture was cooled to RT, filtered through a celite bed and washed with acetone (200 mL).The filtrate was evaporated under reduced pressure to obtain crude product which was dissolved in ethylacetate (500 mL) and washed with water (1 x 100 mL) and brine (1 x 100 mL) and dried over anhy. Na2SO4 to afford crude which was passed through short silica gel column eluting with 10% EtOAc-Hexane to furnish 4-(pentyloxy)benzaldehyde 14 (45 g, 95.3% yield) as a colorless oil. 1H NMR (400 MHz, CDCl3) : δ 9.87 (s, 1H, H-1), 7.82 (d, J = 9.0 Hz, 2H, H-3 & H-3’), 6.99 (d, J = 8.5 Hz, 2H, H-4 & H-4’), 4.03 (t, J = 6.5 Hz, 2H, H-6), 1.82-1.73 (m, 2H, H-7), 1.49-1.37 (m, 4H, H-8 & H-9), 0.94 (t, J = 7.2 Hz, 3H, H-10); 13C NMR (100 MHz, CDCl3) δ 191.0 (C-1), 164.4 (C-5), 132.1 (C-3 & C-3’), 129.9 (C-2), 114.9 (C-4 & C-4’), 68.6 (C-6), 28.9 (C-7), 28.3 (C-8), 22.6 (C-9), 14.2 (C-10); Mass (ESI) m/z= 193.15 [M+H]+
References:
Rao, Pallavi;Hussain, Ismail;Rao, Venkataramanarao;Sen, Saikat;Oruganti, Srinivas [Synthetic Communications,2019,vol. 49,# 17,p. 2180 - 2187] Location in patent:supporting information

123-08-0
900 suppliers
$5.00/10g

5736-91-4
102 suppliers
$40.10/5g
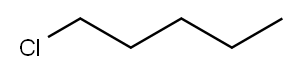
543-59-9
188 suppliers
$20.00/25ml

123-08-0
900 suppliers
$5.00/10g

5736-91-4
102 suppliers
$40.10/5g

2050-04-6
15 suppliers
inquiry

5736-91-4
102 suppliers
$40.10/5g