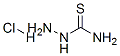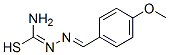
[2-(4-Methoxybenzylidene)hydrazono](amino)methanethiol synthesis
- Product Name:[2-(4-Methoxybenzylidene)hydrazono](amino)methanethiol
- CAS Number:4334-74-1
- Molecular formula:
- Molecular Weight:0
Yield:4334-74-1 92%
Reaction Conditions:
with acetic acid in ethanol at 65; for 2 h;
Steps:
Procedure for the synthesis of and [2-(4-methoxybenzylidene)hydrazine-carbothioamide](L3)36
1.36g (10 mmol) of anisaldehyde and 0.91g (10 mmol) of thiosemicarbazide (1:1 molar ratio) were dissolved in 25 mL ethanol with few drops of acetic acid and the mixture was refluxed at 65°C for 2h. The resulting solution was cooled to room temperature, where upon the microcrystalline products were isolated. The solid mass was washed with diethyl ether and water and dried over fused CaCl2 in a desiccator.
References:
Baruah, Jayantajit;Gogoi, Kongkona;Dewan, Anindita;Borah, Geetika;Bora, Utpal [Bulletin of the Korean Chemical Society,2017,vol. 38,# 10,p. 1203 - 1208] Location in patent:supporting information
123-11-5
839 suppliers
$10.00/10g
methanethiol](/CAS/GIF/4334-74-1.gif)
4334-74-1
6 suppliers
inquiry