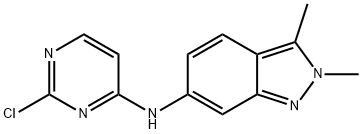
N-(2-Chloropyrimidin-4-YL)-2,3-dimethyl-2H-indazol-6-amine synthesis
- Product Name:N-(2-Chloropyrimidin-4-YL)-2,3-dimethyl-2H-indazol-6-amine
- CAS Number:444731-74-2
- Molecular formula:C13H12ClN5
- Molecular Weight:273.72
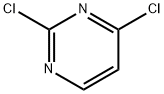
3934-20-1
707 suppliers
$9.00/1g
444731-72-0
214 suppliers
$8.00/1g

444731-74-2
146 suppliers
$11.00/1g
Yield:444731-74-2 90%
Reaction Conditions:
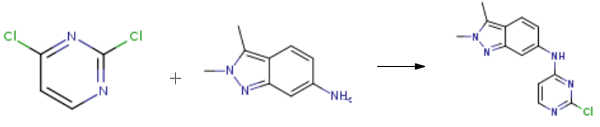
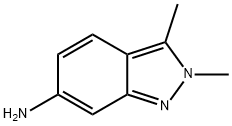
Stage #1: 2,3-dimethyl-2H-indazol-6-aminewith hydrogenchloride in 2-methoxy-ethanol;water at 20;
Stage #2: 2,6-Dichloropyrimidinewith sodium hydrogencarbonate in tetrahydrofuran;ethanol at 77; for 4 h;
Steps:
1
2,3-dimethyl-6-nitro-2H-indazole (0.48g, 2.5mmol, 1 equiv) was dissolved in 2- methoxyethylether (4.3ml) with heating then cooled down to 00C. Tin chloride (1.6g, 7.1 mmol, 2.8 equiv) was added under nitrogen. Concentrated HCl (3.2ml) was added dropwise keeping the temperature below 50C. When all the HCl was added, the reaction was allowed to warm up to room temperature and stirred for 45 min. Ether (14ml) was added and a precipitate was collected too yield the desired product as an hydrochloride salt (0.35g, 86.8%). 1H NMR (d6-DMSO, 400 MHz) δ= 7.67 (d, J = 8Hz, IH), 7.12 (s, I H), 6.79 (d, J = 8Hz, IH), 4.56 (br s, 2H), 2.50 (s, 3H).Preparation of N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (VI) 2,3-dimethyl-2H-imidazole-6-amine HCl (0.58g, 3mmol) was stirred with sodium bicarbonate (1.02g, 12 mmol, 4 equiv) in THF (3ml) and ethanol (12ml). 2,4-
References:
WO2009/62658,2009,A1 Location in patent:Page/Page column 34; 1/10

3934-20-1
707 suppliers
$9.00/1g
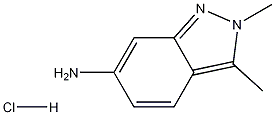
635702-60-2
234 suppliers
$10.00/1g

444731-74-2
146 suppliers
$11.00/1g
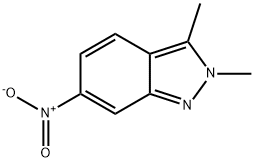
444731-73-1
201 suppliers
$25.00/100mg

444731-74-2
146 suppliers
$11.00/1g
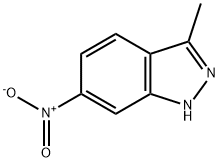
6494-19-5
243 suppliers
$19.00/1g

444731-74-2
146 suppliers
$11.00/1g
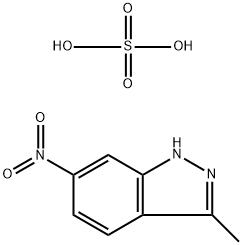
635702-59-9
0 suppliers
inquiry

444731-74-2
146 suppliers
$11.00/1g