
N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine synthesis
- Product Name:N-(2-chloropyriMidin-4-yl)-N,2,3-triMethyl-2H-indazol-6-aMine
- CAS Number:444731-75-3
- Molecular formula:C14H14ClN5
- Molecular Weight:287.75

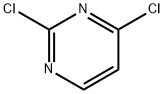
3934-20-1
706 suppliers
$9.00/1g
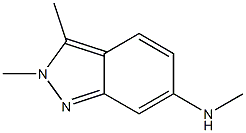
1376676-65-1
23 suppliers
inquiry

444731-75-3
198 suppliers
$49.00/100mg
Yield:444731-75-3 97%
Reaction Conditions:
with Sodium hydrogenocarbonate in water monomer;N,N-dimethyl-formamide at 85; for 3 h;
Steps:
2 Example 2 Preparation of N-(2-chloropyrimidin-4-yl)-.N,2,3-trimethyl-2H-indazol-6-amine (Formula 4)
A flask was charged with N,2,3-trimethyl-2H-indazol-6-amine (1 eq.), 2,4-dichloropyrimidine (1.5 eq.), sodium bicarbonate (2 eq.) and N,N-dimethylformamide. The reaction mixture was stirred at 85 °C until completion of the reaction. Then, water was added and stirred for 3 h. Product crystals were collected by filtration, washed with water and then dried to get title compound as off-white/beige powder (Yield: 97%; LC-purity: 98.5%).
References:
WO2021/162647,2021,A1 Location in patent:Page/Page column 11
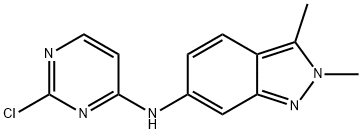
444731-74-2
146 suppliers
$11.00/1g

74-88-4
340 suppliers
$15.00/10g

444731-75-3
198 suppliers
$49.00/100mg

3934-20-1
706 suppliers
$9.00/1g
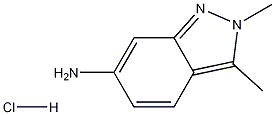
635702-60-2
237 suppliers
$10.00/1g

444731-75-3
198 suppliers
$49.00/100mg

444731-74-2
146 suppliers
$11.00/1g
616-38-6
664 suppliers
$5.00/5G

444731-75-3
198 suppliers
$49.00/100mg

74-88-4
340 suppliers
$15.00/10g

444731-75-3
198 suppliers
$49.00/100mg