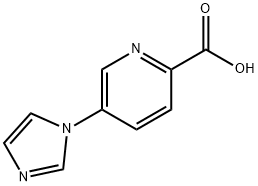
5-(1H-IMidazol-1-yl)picolinic acid synthesis
- Product Name:5-(1H-IMidazol-1-yl)picolinic acid
- CAS Number:1301214-63-0
- Molecular formula:C9H7N3O2
- Molecular Weight:189.17
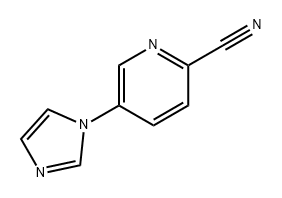
1301214-64-1
0 suppliers
inquiry

1301214-63-0
12 suppliers
inquiry
Yield:1301214-63-0 84%
Reaction Conditions:
Stage #1: 5-(1H-imidazol-1-yl)picolinonitrilewith hydrogenchloride;water for 2 h;Reflux;
Stage #2: with pyridine in water;
Steps:
6 5-(1H-imidazol-1-yl)picolinic acid
5-(1H-imidazol-1-yl)picolinonitrile (136 mg, 0.80 mmol) was heated to reflux in 6N aqueous hydrochloric acid (10 mL) for 2 hours. The reaction mixture was evaporated and the residue was azeotroped with three portions of toluene to give a residue which was purified on an ion exchange column (AG-50 Biorad) eluting with a 0-10% pyridine in water gradient to give the title compound as a white solid (128 mg, 84%). 1H NMR (400 MHz, DMSO-d6) δ ppm 9.10 (s, 1H), 8.50 (s, 1H), 8.26-8.33 (m, 1H), 8.13-8.20 (m, 1H), 7.96 (s, 1H), 7.16 (s, 1H).
References:
US2011/111046,2011,A1 Location in patent:Page/Page column 14
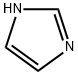
288-32-4
1005 suppliers
$5.00/25g
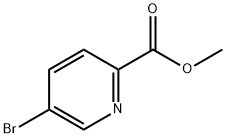
29682-15-3
272 suppliers
$8.00/1g

1301214-63-0
12 suppliers
inquiry
97483-77-7
563 suppliers
$10.00/5g

1301214-63-0
12 suppliers
inquiry