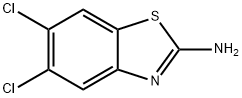
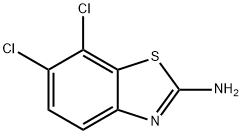
5,6-Dichloro-2-benzothiazolamine synthesis
- Product Name:5,6-Dichloro-2-benzothiazolamine
- CAS Number:24072-75-1
- Molecular formula:C7H4Cl2N2S
- Molecular Weight:219.09
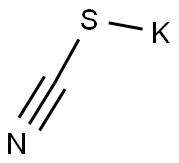
333-20-0
454 suppliers
$13.50/100G
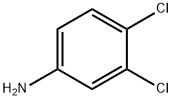
95-76-1
381 suppliers
$10.00/5g

24072-75-1
148 suppliers
$27.00/1g
Yield: 19%
Reaction Conditions:
with bromine;acetic acid at 0 - 15; for 16 h;
Steps:
24B Example 24B: 5 ,6-dichlorobenzo [dlthiazol-2-amine (B-24)
j00320j To a mixture of 3,4-dichloroaniline (10 g, 62 mmol) and potassium thiocyanate (48 g, 0.49 mol) in acetic acid (160 mL) at 0 °C was added slowly with constant stirring a solution of liquidbromine (31 g, 0.19 mol) in acetic acid (160 mL). The temperature was maintained at 0 °C throughout the addition. The solution was stirred for 2 hours at 0°C and 14 hours at 15 °C, then diluted with water (100 mL), adjusted to pH 78 with ammonium hydroxide and extracted with ethyl acetate (3 x 50 mL). The combined organic layers were washed with brine (3 x 300 mL) and concentrated in vacuo. The residue was purified by prep-HPLC [Instmment: GX-B; Column: GEMNI 250 x 50 mm, particle size: 10 .im; Mobile phase: 25-50% acetonitrile in H20 (add 0.1% TFA, v/v)j to give compound B- 24 (2.6 g, 19% yield) as a white solid. LCMS (J): tR=0.694 mi (ES) m/z (M+H)219.0. ‘H-NMR (CD3OD, 400 MHz): 7.89 (s, 1H), 7.55 (s, 1H).
References:
FORUM PHARMACEUTICALS, INC.;ACHARYA, Raksha;BURNETT, Duane, A.;BURSAVICH, Matthew, Gregory;COOK, Andrew, Simon;HARRISON, Bryce, Alden;McRINER, Andrew, J. WO2017/69980, 2017, A1 Location in patent:Paragraph 00319; 00320
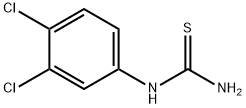
19250-09-0
104 suppliers
$21.00/5g

24072-75-1
148 suppliers
$27.00/1g
25150-27-0
96 suppliers
$60.00/2mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

95-76-1
381 suppliers
$10.00/5g

24072-75-1
148 suppliers
$27.00/1g

25150-27-0
96 suppliers
$60.00/2mg

19250-09-0
104 suppliers
$21.00/5g

24072-75-1
148 suppliers
$27.00/1g

95-76-1
381 suppliers
$10.00/5g

24072-75-1
148 suppliers
$27.00/1g