
5-Bromoindole-2-carboxylic acid methyl ester synthesis
- Product Name:5-Bromoindole-2-carboxylic acid methyl ester
- CAS Number:210345-56-5
- Molecular formula:C10H8BrNO2
- Molecular Weight:254.08
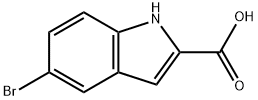
7254-19-5
338 suppliers
$9.00/250mg

210345-56-5
84 suppliers
$75.00/100mg
Yield:-
Reaction Conditions:
Stage #1:5-bromo-2-indolecarboxylic acid with hydrogenchloride in methanol at 20;
Stage #2: with ammonia
Steps:
14 Preparation 14; methyl 5-bromo-1H-indole-2-carboxylate
A solution of 5-Bromo-1H-indole-2-carboxylic acid (commercial, 10.0 g, 41.6 mmol) in methanol (200 ml) was cooled to 0 °C and saturated with HCI (g).. The resulting solution was allowed to warm gradually to room temperature overnight.. The solvent was removed in vacuo and the residue treated with 0.88 ammonia (500 ml).. The resulting solution was extracted with dichloromethane (3-fold 150 ml) and the combined organics dried (magnesium sulphate) and the solvent removed in vacuo to give the required product as a colourless oil (8.35 g).1H NMR (400MHz, CDCl3): δ = 8.96 (1H, bs), 7.83 (1H, s), 7.40 (1H, d), 7.30 (1H, d), 7.14 (1H, s), 3.95 (3H, s). LRMS (electrospray): m/z [M-H]- 252 / 254.
References:
Pfizer Limited EP1460064, 2004, A1 Location in patent:Page 35

67-56-1
775 suppliers
$7.29/5ml-f

7254-19-5
338 suppliers
$9.00/250mg

210345-56-5
84 suppliers
$75.00/100mg
66416-72-6
190 suppliers
$6.00/250mg
55477-80-0
104 suppliers
$130.00/100mg

210345-56-5
84 suppliers
$75.00/100mg
3132-99-8
499 suppliers
$5.00/10g

210345-56-5
84 suppliers
$75.00/100mg
552-89-6
641 suppliers
$5.00/1g

210345-56-5
84 suppliers
$75.00/100mg