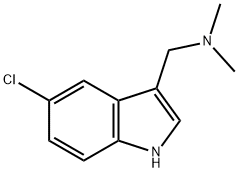
5-Chloroindole-3-acetonitrile synthesis
- Product Name:5-Chloroindole-3-acetonitrile
- CAS Number:81630-83-3
- Molecular formula:C10H7ClN2
- Molecular Weight:190.63
87-52-5
324 suppliers
$16.00/5g
17422-32-1
369 suppliers
$6.00/1g

74-88-4
341 suppliers
$15.00/10g

81630-83-3
31 suppliers
inquiry
Yield:81630-83-3 63%
Reaction Conditions:
with sodium hydroxide;formaldehyd;tetrabutyl ammonium fluoride;acetic acid;dimethyl amine in tetrahydrofuran;dichloromethane;water;ethyl acetate;toluene;
Steps:
24.1 1)
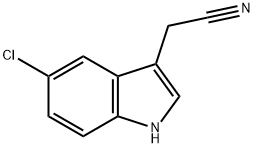
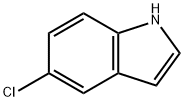
1) Preparation of (5-chloro-1H-indol-3-yl)-acetonitrile To a solution of 5-chloroindole (1.0 g, 6.8 mmol, 1.0 eq.) in a mixture of acetic acid (2 mL) and water (1 mL) were added, at 0°C, formaldehyde (720 μL, 37% in H2O, 8.9 mmol, 1.3 eq) and dimethylamine (1.23 mL, 40% in H2O, 10.9 mmol, 1.7 eq). The mixture was stirred at room temperature for 6 hours and then, quenched with ice and 5 M aqueous sodium hydroxide. The mixture was extracted with dichloromethane and then, the organic layer was dried over MgSO4 and partially evaporated. To the solution gramine in dichloromethane (20 mL) were added, under an argon atmosphere, anhydrous toluene (40 mL) and methyl iodide (824 μL, 13.2 mmol, 2.0 eq.). The mixture was stirred at room temperature for 12 hours and then, concentrated under reduced pressure. To the residue taken up in anhydrous THF (60 mL) were added, under an argon atmosphere, TMSCN (1.25 mL, 9.9 mmol, 1.5 eq.) and TBAF (19.9 mL, 1M, 19.9 mmol, 3.0 eq.). The mixture was stirred at room temperature for 4 hours and then, concentrated under reduced pressure. The crude was taken up in ethyl acetate and the organic layer was washed with aqueous bicarbonate solution and then dried over MgSO4. The solvent was removed under reduced pressure, and the residue purified by silica gel pad filtration (eluent: EtOAc) to afford (5-chloro-1H-indol-3-yl)-acetonitrile as a beige solid (0.80 g, 63%).
References:
EP2266562,2010,A1

50-00-0
850 suppliers
$10.00/25g
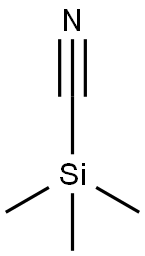
7677-24-9
374 suppliers
$19.00/5g

17422-32-1
369 suppliers
$6.00/1g

81630-83-3
31 suppliers
inquiry
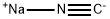
773837-37-9
0 suppliers
inquiry

17422-32-1
369 suppliers
$6.00/1g

74-88-4
341 suppliers
$15.00/10g

81630-83-3
31 suppliers
inquiry

17422-32-1
369 suppliers
$6.00/1g

81630-83-3
31 suppliers
inquiry