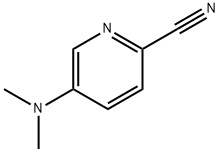
5-(Dimethylamino)picolinonitrile synthesis
- Product Name:5-(Dimethylamino)picolinonitrile
- CAS Number:1159733-63-7
- Molecular formula:C8H9N3
- Molecular Weight:147.18
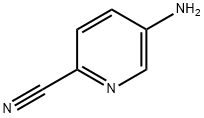
55338-73-3
192 suppliers
$26.00/100mg

74-88-4
342 suppliers
$15.00/10g

1159733-63-7
13 suppliers
inquiry
Yield:1159733-63-7 80%
Reaction Conditions:
with sodium hydride in tetrahydrofuran at 0; for 50 h;
Steps:
(Synthesisof 2-cyano-5-dimethylaminopyridine (11))
5-amino-2-cyanopyridine(10) (1,308mg, 11mmol), iodomethane (CH3I) (2.0ml, 33mmol) were dissolved in tetrahydrofuran (THF) (100ml). The solution was brought to 0°C and 60% sodium hydride (NaH) (1308 mg, 33mmol) was added slowly and the mixture was stirred for 50 hours. A small amount of methanol was added to the reaction mixture to decompose excess ofiodomethane, sodium hydride. It was followed by adding water (10ml) andextracted with ethyl acetate (3 × 30ml). The organic layers were combined and dried over anhydrous sodium sulfate, and then concentratedunder reduced pressure. The obtained residue was purified by columnchromatography [silica gel 59.2 g; hexane - ethyl acetate (1: 2)] to obtain2-cyano-5-dimethylaminopyridine (11) (1,303.8mg, 8. 86mmol, 80%) as a brown powder solid.
References:
JP2015/193584,2015,A Location in patent:Paragraph 0066; 0067
327056-62-2
254 suppliers
$5.00/1g

124-40-3
498 suppliers
$18.00/100ml

1159733-63-7
13 suppliers
inquiry

50-00-0
845 suppliers
$10.00/25g

55338-73-3
192 suppliers
$26.00/100mg

1159733-63-7
13 suppliers
inquiry