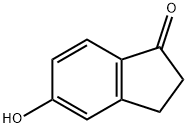
5-Hydroxy-1-indanone synthesis
- Product Name:5-Hydroxy-1-indanone
- CAS Number:3470-49-3
- Molecular formula:C9H8O2
- Molecular Weight:148.16
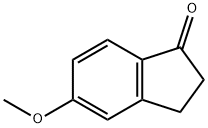
5111-70-6
230 suppliers
$34.00/5g

3470-49-3
164 suppliers
$10.00/1g
Yield:3470-49-3 90%
Reaction Conditions:
AlCl3 in benzene
Steps:
6 (1S,5R,6S,8R)-6-(1-Hydroxyethyl)-2-(1-indanone-5-yloxy-methyl)-1methylcarbapenem-3-carboxylic acid sodium salt
The starting material was prepared as follows: To a solution of 5-methoxy-1-indanone (2 g, 12.3 mmol) in benzene (50 ml) was added AlCl3 (4 g, 31 mmol). The mixture was heated at reflux for 5 hours and extracted with ethyl acetate. The organic phase was evaporated and purified by flash chromatography, eluding with CH2 Cl2 /CH3 CN (90/10) to give 5-hydroxy-1-indanone as a yellow solid (1.65 g; 90%). 1 H-NMR (DMSO-d6): δ 2.58-2.67 (m, 2H); 2.94-3.01 (m, 2H); 6.79 (dd, 1H), 6.84 (s, 1H); 7.4.7 (d, 1H).
References:
Zeneca Limited;Zeneca Pharma SA US5607928, 1997, A
35999-20-3
102 suppliers
$70.00/100mg

3470-49-3
164 suppliers
$10.00/1g
621-54-5
185 suppliers
$9.00/250mg

3470-49-3
164 suppliers
$10.00/1g
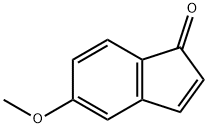
72913-59-8
12 suppliers
$100.00/100mg

3470-49-3
164 suppliers
$10.00/1g
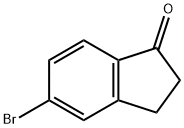
34598-49-7
367 suppliers
$14.00/5g

3470-49-3
164 suppliers
$10.00/1g