
5-METHOXY-1H-INDAZOLE synthesis
- Product Name:5-METHOXY-1H-INDAZOLE
- CAS Number:94444-96-9
- Molecular formula:C8H8N2O
- Molecular Weight:148.16
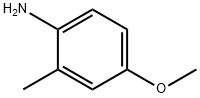
102-50-1
217 suppliers
$6.00/5g

94444-96-9
134 suppliers
$24.00/250mg
Yield: 18%
Reaction Conditions:
with acetic acid;sodium nitrite in water at 20;
Steps:
4.a Reference Example 4; Synthesis of 1H-indazol-5-ol; (a) Synthesis of 5-methoxy-1H-indazole
A solution of sodium nitrite (3.38 g, 49.0 mmol) in water (8.1 ml) was added to a solution of 4-methoxy-2-methylaniline (6.69 g, 48.8 mmol) in acetic acid (350 ml) in an ice-water bath while maintaining the temperature at 25°C or lower, and stirred overnight at room temperature. Then, the reaction solution was poured into water and extracted with chloroform. The organic layer was washed with a saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate and then distilled under reduced pressure to remove the solvent, and the resulting residue was purified by a silica gel column chromatography (eluent: chloroform/methanol = 9/1) to obtain 5-methoxy-1H-indazole (1.30 g, 18%).1H-NMR (DMSO-d6) δ; 3.76 (3H, s), 6.98 (1H, dd, J=8.8, 1.8Hz), 7.15 (1H, d, J=1.8Hz), 7.42 (1H, d, J=8.8Hz), 7.93 (1H, s), 12.89 (1H, brs).
References:
Sumitomo Pharmaceuticals Company, Limited EP1403255, 2004, A1 Location in patent:Page 44
31601-41-9
41 suppliers
$155.00/10g

94444-96-9
134 suppliers
$24.00/250mg
672-13-9
267 suppliers
$29.19/1g

94444-96-9
134 suppliers
$24.00/250mg
105728-90-3
173 suppliers
$12.00/1g

94444-96-9
134 suppliers
$24.00/250mg
459-60-9
344 suppliers
$7.00/5g

94444-96-9
134 suppliers
$24.00/250mg