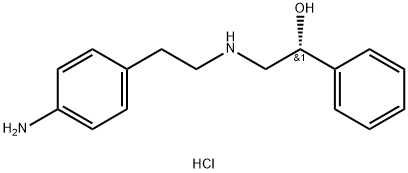
(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride synthesis
- Product Name:(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride
- CAS Number:521284-22-0
- Molecular formula:C16H21ClN2O
- Molecular Weight:292.81

A mixture of (100 g) (R)-2-[2’ -(4-nitrophenyl)ethyl]amino] -1 -phenylethanol monohydroch bride (prepared according to example Ib), methanol (2000 mL) and Raney Nickel (20 g, wet) was stirred under hydrogen pressure (60 psi) for 6 hrs. The reaction solution was filtered, and the filtrate was concentrated in vacuo. The residue was mixed with isopropanol (300 mL) and reaction mixture was heated to 78°C (±2). The mixture was added to toluene (900 mL). The reaction mixture was gradually cooled to 28°C (±2) and stirred at this temperature for 3 hrs. The solid obtained was filtered, washed with toluene and dried under vacuo at 48°C (±2) to obtain (R)-2-[[2-(4-aminophenyl)ethyl]-amino]- I -phenylethanol monohydrochioride.Yield: 78.1 g (86.1%); Purity by HPLC: 99.14 %.
![BenzeneMethanol, -[[[2-(4-nitrophenyl)ethyl]aMino]Methyl]-, (R)-](/CAS/GIF/521284-21-9.gif)
521284-21-9
171 suppliers
inquiry
![(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride](/CAS/GIF/521284-22-0.gif)
521284-22-0
187 suppliers
$10.00/250mg
Yield:521284-22-0 93%
Reaction Conditions:
with ammonia hydrochloride;zinc in tetrahydrofuran;lithium hydroxide monohydrate at 20; for 1 h;Reagent/catalyst;Time;
Steps:
1-3 Example 1. (R)-2-((4-aminophenethyl)amino)-1-phenylethanol (Formula 1) Synthesis Example
(R)-2-{[2-(4-nitrophenyl)ethyl]amino}-1-phenylethanol-hydrochloride (Formula 2) 10 g of tetrahydrofuran (THF) 25 , and after adding 80 ml of purified water, 10 g of ammonium chloride (NH 4 Cl) was added. Add 20 g of zinc powder in portions After that, the reaction was carried out at room temperature for 1 hour. After completion of the reaction, the zinc powder was removed by filtration. Ethyl acetate in the filtrate 50 ml was added, and an aqueous NaOH solution was added to adjust the pH. Concentrated hydrochloric acid was added to the obtained organic layer, and then cooled to 0-5 °C. Each crystallized with stirring for 1 hour. The resulting crystals were filtered, washed with ethyl acetate, and dried under reduced pressure at room temperature. Thus, 8.4 g of Chemical Formula 1 was obtained (yield 93%, purity 99.91%, compound VII was not detected).
References:
KR2021/73972,2021,A Location in patent:Paragraph 0064-0068
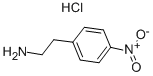
29968-78-3
424 suppliers
$18.00/5g
![(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride](/CAS/GIF/521284-22-0.gif)
521284-22-0
187 suppliers
$10.00/250mg
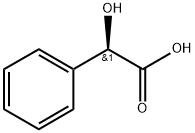
611-71-2
620 suppliers
$13.00/5g
![(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride](/CAS/GIF/521284-22-0.gif)
521284-22-0
187 suppliers
$10.00/250mg
![(alphaR)-alpha-Hydroxy-N-[2-(4-nitrophenyl)ethyl]benzeneacetamide](/CAS/GIF/521284-19-5.gif)
521284-19-5
154 suppliers
$7.00/250mg
![(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride](/CAS/GIF/521284-22-0.gif)
521284-22-0
187 suppliers
$10.00/250mg