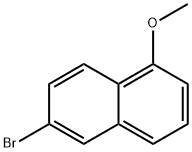
6-bromo-1-methoxynaphthalene synthesis
- Product Name:6-bromo-1-methoxynaphthalene
- CAS Number:54828-63-6
- Molecular formula:C11H9BrO
- Molecular Weight:237.09
91270-68-7
76 suppliers
$45.00/10mg

74-88-4
342 suppliers
$15.00/10g

54828-63-6
32 suppliers
inquiry
Yield: 75%
Reaction Conditions:
with potassium carbonate in acetone at 22 - 65; for 18 h;
Steps:
1 Step 1: Synthesis of 6-bromo-1-methoxynaphthalene (2)
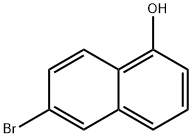
6-Bromonaphthalen-1-ol (compound 1, 10 g, 44.8 mmol) was dissolved in dry acetone (180 mL) under a room temperature (-22° C.) atmosphere in a 500 mL, 3-necked round bottomed flask topped with a reflux condenser.
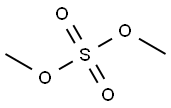
Then, potassium carbonate (12.39 g, 90 mmol) and methyl iodide (5.61 ml, 90 mmol) were added and the reaction mixture was stirred at 65° C. for 18 hours.
The reaction was cooled to room temperature (-22° C.), causing a white solid to precipitate from the reaction mixture.
The precipitate was then was filtered off and the filtrate was concentrated under vacuum.
The reaction mixture was partitioned between EtOAc and brine (200 mL) and the organics were separated and washed with brine (2*50 mL), dried over MgSO4, and the solvents removed to afford an orange oil.
Recrystallization from iso-hexane afforded compound 2 of Scheme I as a white solid (8.0 g, 33.7 mmol, 75% yield).
References:
Universal Display Corporation;FELDMAN, Jerald;BOUDREAULT, Pierre-Luc T. US2019/88887, 2019, A1 Location in patent:Paragraph 0152; 0153

91270-68-7
76 suppliers
$45.00/10mg
77-78-1
292 suppliers
$19.69/1ml

54828-63-6
32 suppliers
inquiry