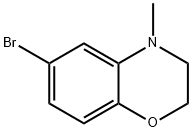
6-Bromo-4-methyl-3,4-dihydro-2H-1,4-benzoxazine synthesis
- Product Name:6-Bromo-4-methyl-3,4-dihydro-2H-1,4-benzoxazine
- CAS Number:188947-79-7
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
![6-Bromo-3,4-dihydro-2H-benzo[1,4]oxazine hydrochloride](/CAS/GIF/105655-01-4.gif)
105655-01-4
122 suppliers
$27.00/250mg

74-88-4
342 suppliers
$15.00/10g

188947-79-7
28 suppliers
$65.00/25mg
Yield:188947-79-7 87%
Reaction Conditions:
with potassium carbonate in acetonitrile at 50;Inert atmosphere;
Steps:
1 [Synthesis of Compound E5]
[Synthesis of Compound E5] (0136) Compound E4 (1.90 g, 8.88 mmol, 1 Eq) was dissolved in acetonitrile (20 mL) in an argon atmosphere, and potassium carbonate (1.98 g, 14.3 mmol, 1.6 Eq) and methyl iodide (3.0 mL, 47.5 mmol, 5 Eq) were added and the combination was stirred overnight at 50° C. After returning the reaction system to room temperature and distilling off the solvent under reduced pressure, the reaction system was diluted by ethyl acetate, the insoluble matter was distilled off by filtration under reduced pressure, and the filtrate was removed under reduced pressure. The residue obtained was purified by medium-pressure silica gel chromatography (eluent: n-hexane/ethyl acetate=98/2 to 90/10), and the target compound E5 (1.77 g, 87%) was obtained as a pale yellow solid. (0137) 1H NMR (CDCl3): δ 6.73-6.70 (m, 2H), 6.61 (d, J=8.0 Hz, 1H), 4.25 (t, J=4.4 Hz, 2H), 3.26 (t, J=4.4 Hz, 2H), 2.86 (s, 3H).; 13C NMR (CDCl3): δ 143.3 (C), 137.9 (C), 120.5 (CH), 117.2 (CH), 114.9 (CH), 113.8 (C), 64.8 (CH2), 48.8 (CH2), 38.7 (CH3).; HRMS-ESI (m/z): [M+H]+ calcd for C9H11BrNO: 228.00185. found: 228.00185. found: 228.00168 (0.2 mDa, 0.8 ppm).
References:
US2017/45525,2017,A1 Location in patent:Paragraph 0135; 0136; 0137

50-00-0
850 suppliers
$10.00/25g
![6-Bromo-3,4-dihydro-2H-benzo[1,4]oxazine hydrochloride](/CAS/GIF/105655-01-4.gif)
105655-01-4
122 suppliers
$27.00/250mg

188947-79-7
28 suppliers
$65.00/25mg
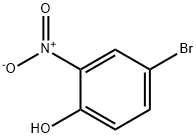
7693-52-9
232 suppliers
$5.00/1g

188947-79-7
28 suppliers
$65.00/25mg
![Acetamide, N-[2-(acetyloxy)-5-bromophenyl]-](/CAS/20210305/GIF/36749-01-6.gif)
36749-01-6
0 suppliers
inquiry

188947-79-7
28 suppliers
$65.00/25mg

40925-68-6
255 suppliers
$6.00/1g

188947-79-7
28 suppliers
$65.00/25mg