
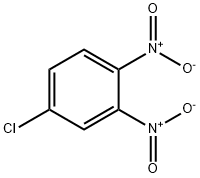
4-Chloro-2-(methylthio)nitrobenzene, 4-Chloro-2-(methylsulphanyl)nitrobenzene, 4-Chloro-2-(methylmercapto)nitrobenzene synthesis
- Product Name:4-Chloro-2-(methylthio)nitrobenzene, 4-Chloro-2-(methylsulphanyl)nitrobenzene, 4-Chloro-2-(methylmercapto)nitrobenzene
- CAS Number:70019-41-9
- Molecular formula:C7H6ClNO2S
- Molecular Weight:203.65

700-37-8
207 suppliers
$9.00/1g
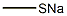
5188-07-8
265 suppliers
$13.00/5g

70019-41-9
14 suppliers
$87.00/250mg
Yield:70019-41-9 100%
Reaction Conditions:
in methanol at 15 - 25;
Steps:
A.144 Example 144; N-[4-methoxy-2-(methylthio)phenyl]-N'-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl]urea.
Sodium thiomethoxide (0.75 g, 10.7 mmol) is added dropwise as a solution in MeOH (25 mL) to 5-chloro-2-fluoronitrobenzene (1.9 g, 10.7 mmol) in MeOH (50 mL) at RT. The yellow-green solution is stirred for 2 h and then quenched with 1M citric acid (50 mL). The MeOH is removed and the residue is dissolved in EtOAc (50 mL) and washed with 1M citric acid (50 mL), 0.5M NaOH (50 mL), and brine (50 mL). The organics are separated, dried (MgSO4), and the solvent is removed under reduced pressure to give 4-chloro-2-thiomethylnitrobenzene (2.2 g, 100% yield) as a yellow solid. Sodium (0.25 g, 10.7 mmol) is added in small portions to MeOH (25 mL). The solution of NaOMe is then added to 4-chloro-2-thiomethylnitrobenzene (2.2 g, 10.7 mmol) in MeOH (50 mL) at RT. The reaction is heated under reflux for 24 h and cooled to RT. A second equivalent of NaOMe is added and the reaction is heated under reflux for an additional 6 h. The reaction is quenched with 1M citric acid (50 mL) and H2O (50 mL). The MeOH is removed under reduced pressure and the aqueous is extracted with EtOAc (3×50 mL), dried (MgSO4), and the solvent is removed. A 1:1 mixture of product:starting material remained. The material is dissolved in DMF (50 mL) and NaH (0.65 eq, 60% oil disp.) and MeOH (0.65 eq) are added and the reaction is heated to reflux for 24 h. The work up is repeated to give 4-methoxy-2-(methylthio)-1-nitrobenzene (91.8 g, 85% yield) as a dark yellow solid. 4-Methoxy-2-(methylthio)-1-nitrobenzene is dissolved in minimal EtOAc (5 mL) and diluted with EtOH (50 mL). 10% Pd/C catalyst is added as a slurry in EtOAc and the mixture put on the Parr apparatus in the presence of H2 (44 psi to 32 psi) for 0.5 h. The reaction mixture is filtered over Celite to remove the catalyst and the solvent is removed to give 4-methoxy-2-(methylthio)aniline (0.26G, 61% yield) as an orange oil. 4-Methoxy-2-(methylthio)aniline (0.26 g, 1.5 mmol) is added dropwise as a solution in EtOAc (25 mL) to phosgene (13.0 mL, 20% solution in toluene) in EtOAc (50 mL). After complete addition, the reaction is heated under reflux for 0.5 h. The reaction is cooled to RT and the solvent is removed under reduced pressure to give 1-isocyanato-4-methoxy-2-(methylthio)benzene (0.29 g, 97% yield) as a brown oil. Example 144 is obtained using the isocyanate according to Method A, making non-critical variations. Yield 55%. HRMS calcd for C12H11F3N4O2S2+H 365.0354 found 365.0347.
References:
US2003/236287,2003,A1 Location in patent:Page 34

2516-95-2
311 suppliers
$5.00/5g
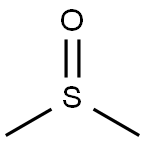
67-68-5
1211 suppliers
$15.00/100g

70019-41-9
14 suppliers
$87.00/250mg
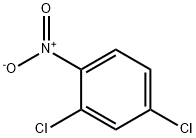
611-06-3
7 suppliers
$15.00/25g
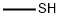
74-93-1
120 suppliers
$454.02/5MG

70019-41-9
14 suppliers
$87.00/250mg
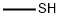
74-93-1
120 suppliers
$454.02/5MG
610-40-2
102 suppliers
$50.00/10g

70019-41-9
14 suppliers
$87.00/250mg