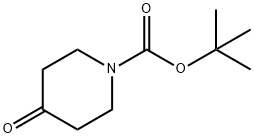
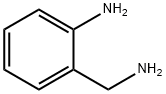
tert-butyl 4-(2-aMinobenzylaMino)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-aMinobenzylaMino)piperidine-1-carboxylate
- CAS Number:79098-98-9
- Molecular formula:C17H27N3O2
- Molecular Weight:305.42
Yield:79098-98-9 94%
Reaction Conditions:
with sodium tris(acetoxy)borohydride in dichloromethane at 20; for 48 h;
Steps:
2-Amino-N-(1-Boc-piperidin-4-yl)benzyl amine (4a) Preparation:
To a 500 mL round-bottom flask was added 2-aminobenzylamine (5.00 g, 40.0 mmol) and DCM(200 mL). The solution was stirred at room temperature and N-Boc-4-piperidone (8.20 g, 40.9mmol) was added, followed by sodium triacetoxyborohydride (13.4 g, 61.4 mmol). The solution was stirred continuously for 48 h at which point the TLC indicated the reaction was complete. Thereaction mixture was filtered and the filtrate washed twice with 1.0 M NaOH (aq.). The organiclayer was dried (Na2SO4 anh.), filtered, and concentrated to yield 4a which was used withoutfurther purification.Physical State: yellow foam (11.6 g, 94 %)
References:
Habay, Stephen A.;Miller, Julia M.;Bowler, Matthew M.;Manchak, Randi;Thomas, John Z. [Tetrahedron Letters,2018,vol. 59,# 36,p. 3389 - 3391] Location in patent:supporting information
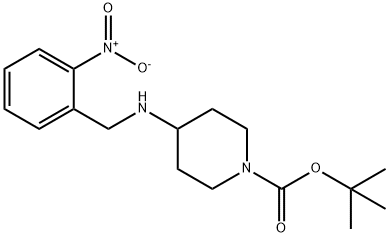
87120-79-4
19 suppliers
$309.00/1g

79098-98-9
14 suppliers
inquiry
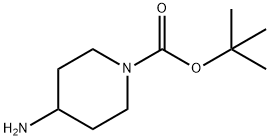
87120-72-7
0 suppliers
$6.00/1g

79098-98-9
14 suppliers
inquiry
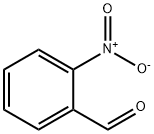
552-89-6
638 suppliers
$5.00/1g

79098-98-9
14 suppliers
inquiry