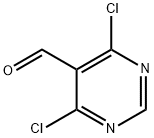
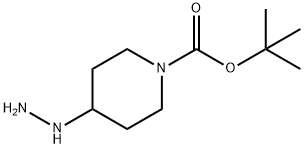
tert-butyl 4-(4-chloro-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(4-chloro-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate
- CAS Number:791852-35-2
- Molecular formula:C15H20ClN5O2
- Molecular Weight:337.8046
Yield:791852-35-2 70%
Reaction Conditions:
Stage #1: 4-(N'-tert-Butoxycarbonyl-hydrazino)-piperidine-1-carboxylic acid tert-butyl ester;2,6-dichloro-pyrimidine carbaldehydewith N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 0 - 20;
Stage #2: in toluene at 110; for 6 h;
Steps:
19.C
Method C; A solution of 4-(tert-butoxycarbonyl-hydrazino)-piperidine-1-carboxylic acid tert-butyl ester (Intermediate 15; 1.80 g, 5.71 mmol), diisopropylethylamine (2.0 mL, 11.3 mmol), and 4,6-dichloropyrimidine-5-carbaldehyde (Intermediate 16; 1.00 g, 5.65 mmol) in tetrahydrofuran (10 mL) was stirred at 0° C. for 30 min, then it was warmed up to rt and stirred for 4 h. The solid was filtered. The filtrate was washed with brine (10 mL) and the aqueous layer was extracted with methyl tert-butyl ether (1×10 mL). The combined organic layers were washed with brine (1×10 mL) and concentrated to give an orange-red semi-solid. Toluene (10 mL) was added and the solution was heated to 110° C. for 6 h. The reaction mixture was cooled to room temperature and concentrated to give a semi-solid. This crude solid was dissolved in the minimum amount of dichloromethane and passed through a pad of silica gel, eluting with 9% ethyl acetate/hexanes. Concentration of fractions homogeneous for the product gave 4-(4-chloro-pyrazolo[3,4-d]pyrimidin-1-yl)-piperidine-1-carboxylic acid tert-butyl ester (1.33 g, 70%) as a cream white solid. 1H NMR (CDCl3) δ 1.49 (s, 9H), 1.9-2.1 (m, 2H), 2.25 (qd, 2H, J=12.6, 4.5 Hz), 2.97 (t, 2H, J=12.0 Hz), 4.30 (br, 2H), 4.96 (tt, 1H, J=11.4, 4.2 Hz), 8.15 (s, 1H), 8.75 (s, 1H).
References:
US2009/286812,2009,A1 Location in patent:Page/Page column 24
![4-Chloro-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/5399-92-8.gif)
5399-92-8
232 suppliers
$13.43/250mgs:

109384-19-2
478 suppliers
$5.00/1g
![tert-butyl 4-(4-chloro-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate](/CAS/20180703/GIF/791852-35-2.gif)
791852-35-2
9 suppliers
inquiry
87120-72-7
28 suppliers
$6.00/1g
![4-Chloro-1H-pyrazolo[3,4-d]pyrimidine](/CAS/GIF/5399-92-8.gif)
5399-92-8
232 suppliers
$13.43/250mgs:
![tert-butyl 4-(4-chloro-1H-pyrazolo[3,4-d]pyrimidin-1-yl)piperidine-1-carboxylate](/CAS/20180703/GIF/791852-35-2.gif)
791852-35-2
9 suppliers
inquiry
![4-[2-[(1,1-dimethylethoxy)carbonyl]hydrazinyl]-1-Piperidinecarboxylic acid 1,1-dimethylethyl ester](/CAS/20180703/GIF/380226-99-3.gif)