
BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]- synthesis
- Product Name:BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]-
- CAS Number:817204-35-6
- Molecular formula:C17H19N3O6
- Molecular Weight:361.3493
Yield:817204-35-6 53%
Reaction Conditions:
in diethyl ether at -78; for 2 h;Inert atmosphere;
Steps:
7.c c. Preparation of compound 703.
c. Preparation of compound 703. Compound 702 (48.57 g, 8.26 mmol) was dissolved in anhydrous toluene (~400 mL) and the solvent was removed in vacuo with exclusion of moisture. The residue was then further dried in vacuo (oil pump) for another 2 hours. With strict exclusion of moisture, the residual foam was dissolved in anhydrous diethyl ether (1.03 L) under argon. The resulting solution was cooled to -78°C under argon and MeLi (1.6 M, 258.0 mL, 0.413 mol) was added dropwise via additional funnel. After the addition was complete, the mixture was stirred for 2 hours at -78°C. Aqueous 1 M NH4CI (500 mL) was added slowly. After warming to room temperature, the mixture was washed with H20 (2 x 500 mL), dried (Na2S04), and then concentrated to dryness to give a brown foam (-60 g, >100%). The reaction was performed two more times using 37.62 g and 56.4 g of compound 702. The combined crude products (128.0 g, 0.212 mol) were dissolved in THF (1.28 L) and treated with coned HOAc (23 mL, 0.402 mol). To the solution was added TBAF (384.0 mL, 1 M in THF). The solution was stirred at room temperature for 0.75 hours and the mixture was treated with silica gel (750 g) and concentrated to dryness. The powder was placed on a silica gel column packed in CH2CI2. Elution with 1 :7 EtOH-CH2CI2 afforded a dark waxy solid that was pre-adsorbed on silica gel (300 g) and chromatographed as before. Compound 703 (46.4 g, 53.0 % from 702) was isolated as an off-white solid. 1H NMR (DMSO-d6): δ 1.20 (s, 3H, CH3), 3.62-3.69 (m, 2H,), 3.73-3.78 (m, 2H,), 5.19 (t, 1H, J = 5.4 Hz, OH-5'), 5.25 (s, 1 H, OH-2'), 5.52 (d, 1 H, J = 5.0 Hz, OH-3'), 5.99 (s, 1 H, H-1 '), 7.32 (d, 1 H, J = 5.8 Hz), 7.50 (Ψ, 2H, J =7.7 Hz), 7.62 (Ψ, 1 H, J =7.3 Hz), 8.00 (d, 2H, J =7.3 Hz), 8.14 (d, 1H, J =6.9 Hz), 11.22 (s, 1 H, NH). Anal. Calcd for C17H19N306 · 0.5 H20: C, 55.13; H, 5.44; N, 11.35. Found: C, 55.21 ; H, 5.47; N, 11.33.
References:
WO2013/40492,2013,A2 Location in patent:Page/Page column 93-94
![BenzaMide, N-[1,2-dihydro-1-[2-C-Methyl-3,5-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-β-D-arabinofuranosyl]-2-oxo-4-pyriMidinyl]-](/CAS/20180808/GIF/817204-34-5.gif)
817204-34-5
5 suppliers
inquiry
![BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]-](/CAS/20180808/GIF/817204-35-6.gif)
817204-35-6
1 suppliers
inquiry
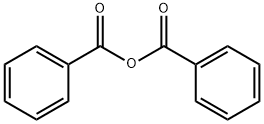
93-97-0
382 suppliers
$6.00/25g
![BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]-](/CAS/20180808/GIF/817204-35-6.gif)
817204-35-6
1 suppliers
inquiry

13089-48-0
255 suppliers
$16.00/1g
![BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]-](/CAS/20180808/GIF/817204-35-6.gif)
817204-35-6
1 suppliers
inquiry

65-46-3
706 suppliers
$5.00/1g
![BenzaMide, N-[1,2-dihydro-1-(2-C-Methyl-β-D-arabinofuranosyl)-2-oxo-4-pyriMidinyl]-](/CAS/20180808/GIF/817204-35-6.gif)
817204-35-6
1 suppliers
inquiry
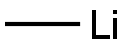
![Cytidine, N-benzoyl-2^-deoxy-2^-oxo-3^,5^-O-[1,1,3,3-tetrakis(1-Methylethyl)-1,3-disiloxanediyl]-](/CAS/20180808/GIF/119411-03-9.gif)