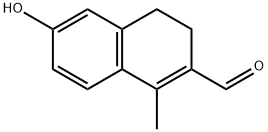
2-Naphthalenecarboxaldehyde, 3,4-dihydro-6-hydroxy-1-methyl- synthesis
- Product Name:2-Naphthalenecarboxaldehyde, 3,4-dihydro-6-hydroxy-1-methyl-
- CAS Number:847585-92-6
- Molecular formula:C12H12O2
- Molecular Weight:188.22
847585-91-5
0 suppliers
inquiry

847585-92-6
1 suppliers
inquiry
Yield:-
Reaction Conditions:
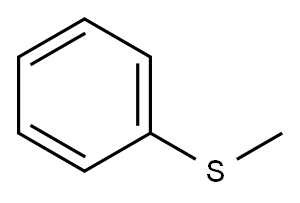
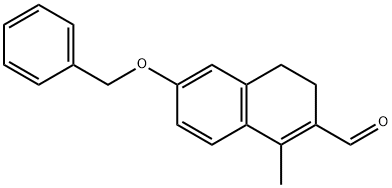
Stage #1: 6-(benzyloxy)-1-methyl-3,4-dihydronaphthalene-2-carbaldehydewith trifluoroacetic acid in methyl-phenyl-thioether at 0 - 20; for 4 h;
Stage #2: with sodium hydroxide in methyl-phenyl-thioether;water at 0;
Steps:
13
Example 13 6-hydroxy-1-methyl-3,4-dihydro-2-naphthalenecarbaldehyde: To thioanisole (35 mL), trifluoroacetic acid (140 mL) was added at 0°C. Then, the compound (9.17 g) prepared in Example 12 was added in portions thereto, followed by stirring at room temperature for 4 hours. The reaction mixture was poured into ice, followed by adding a 5N aqueous sodium hydroxide solution. After washing with tert-butyl methyl ether, 1N hydrochloric acid was added to the aqueous layer, followed by extracting with ethyl acetate. The organic layer was dried and concentrated. The obtained residue was purified by silica gel column chromatography (hexane:ethyl acetate = 5:1 to 2:1) to thereby give the title compound (6.03 g) having the following physical properties. TLC: Rf 0.26 (hexane: ethyl acetate = 3:1); ESI-MS: 189 (M+H)+; NMR (CDCl3): δ 10.31 (s, 1H), 7.45 (d, J = 8.4 Hz, 1H), 6.76 (dd, J = 8.4, 2.6 Hz, 1H), 6.70 (d, J = 2.6 Hz, 1H), 2.66-2.75 (m, 2H), 2.50 (s, 3H), 2.46-2.55 (m, 2H).
References:
EP1760071,2007,A1 Location in patent:Page/Page column 50
3470-50-6
222 suppliers
$6.00/1g

847585-92-6
1 suppliers
inquiry
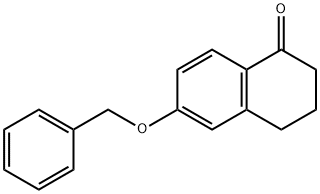
32263-70-0
105 suppliers
$18.00/100mg

847585-92-6
1 suppliers
inquiry
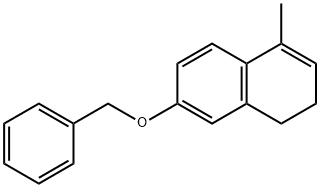
847585-90-4
1 suppliers
inquiry

847585-92-6
1 suppliers
inquiry