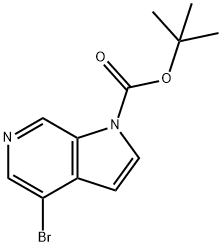
tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate
- CAS Number:850892-97-6
- Molecular formula:C12H13BrN2O2
- Molecular Weight:297.15

24424-99-5
850 suppliers
$13.50/25G
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
168 suppliers
$18.00/100mg
![tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/GIF/850892-97-6.gif)
850892-97-6
16 suppliers
inquiry
Yield:850892-97-6 93%
Reaction Conditions:
with pyridine in dichloromethane at 20; for 3 h;Inert atmosphere;
Steps:
Intermediate 136: te,butvI 4-bromo-1H-pyrrolor2,3-clpyridine-1-ca rboxvlate
To a solution of pyridine (1.210 mL, 14.96 mmol) in dichloromethane (5mL) under nitrogen was added 4-bromo-1H-pyrrolo[2,3-c]pyridine (2.68 g, 13.60 mmol) (commercially available from Aldrich) anddi-ter1butyl dicarbonate (3.27 g, 14.96 mmol). The reaction mixture was stirred at room temperature for 3 hours. 5mL of 2M HCI was added and the organic phase was separated. The aqueous phase was exctracted with further portions of DCM (2 x 5mL). The combined organic phases were dried by filtering through a hydrophobic frit and then concentrated in vacuo to give ter1butyl 4-bromo-1H- pyrrolo[2,3-c]pyridine-1-carboxylate (4.19 g, 12.69 mmol, 93 % yield)LCMS (2 mins Formic) Peak Rt = 1.15 minutes, m/z= 297, 299 for [MH]
References:
WO2017/202742,2017,A1 Location in patent:Page/Page column 101

6627-89-0
147 suppliers
$8.00/5g
![4-bromo-1H-pyrrolo[2,3-c]pyridine](/CAS/GIF/69872-17-9.gif)
69872-17-9
168 suppliers
$18.00/100mg
![tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/GIF/850892-97-6.gif)
850892-97-6
16 suppliers
inquiry
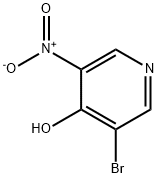
31872-65-8
139 suppliers
$12.00/250mg
![tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/GIF/850892-97-6.gif)
850892-97-6
16 suppliers
inquiry
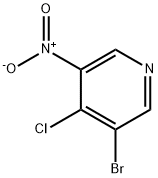
31872-63-6
178 suppliers
inquiry
![tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/GIF/850892-97-6.gif)
850892-97-6
16 suppliers
inquiry

69872-15-7
119 suppliers
inquiry
![tert-Butyl 4-broMo-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/GIF/850892-97-6.gif)
850892-97-6
16 suppliers
inquiry