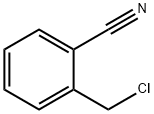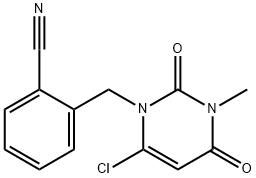
2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile synthesis
- Product Name:2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile
- CAS Number:865758-96-9
- Molecular formula:C13H10ClN3O2
- Molecular Weight:275.69

74-88-4
351 suppliers
$15.00/10g
![2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile](/CAS/GIF/865758-96-9.gif)
865758-96-9
309 suppliers
inquiry
Yield:865758-96-9 72%
Reaction Conditions:
Stage #1:2-[(6-chloro-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)methyl]benzonitrile with sodium hydride;lithium bromide in tetrahydrofuran;DMF (N,N-dimethyl-formamide) at 0 - 20; for 0.333333 h;
Stage #2:methyl iodide in tetrahydrofuran;DMF (N,N-dimethyl-formamide) at 20 - 35;
Steps:
To a cold (0 °C) solution ofbenzylated 6-chlorouracil 2 (10 g, 38 mmol) in DMF-THF (1:1, 300 mL) under nitrogen, was added NaH (60%, 1.6 g, 39.9 mmol) in portions, followed by adding LiBr (2g). The mixture was stirred at r.t for 20 min. After adding iodomethane (5.4 mL, 76 mmol), the flask was sealed and stirred at this temperature for 10 min, rt for 2h, and 35 °C overnight, and then concentrated in vacuo. It will be understood that alkylation of the amine may be performed under standard conditions known in the art, including the use of a base such as NaH, LiH or the like in an organic solvent or mixture of solvents. The solvent may include DMSO, THF, DMF and the like, or mixtures thereof. In addition, additives may be used, including LiBr, LiI, NaI and the like. For example, the alkylation can be performed using methyliodide and KsCO3 in acetone. The reaction may be performed at about 15-45 °C, preferably at about 20-43 °C, and more preferably at about 35-41 °C until the reaction is complete. The residue was dissolved in CHCl3 and washed with water and brine, dried (Na2SO4), and filtered then concentrated in vacuo. The crude product was crystallized from THF-Hexanes to give 7.6 g (72%) of the title compound 3. It will also be understood by those skilled in the art that the benzonitrile may be purified in a variety of organic solvents or solvent mixtures. For example, the benzonitrile can be purified by adding a mixture of dichloromethane and heptane. Optionally, the benzonitrile may be further purified in an organic solvent or mixture of solvents such as dichloromethane, chloroform, acetonitrile, THF, ethyl acetate, isopropyl acetate and the like. Preferably, the product is purified and washed with ethyl acetate. 1H NMR (400 MHz, DMSO): δ 7.87 (d, 1H, J = 7.6 Hz), 7.70 (t, 1H, J = 7.6 Hz), 7.51 (t, 1H, J = 7.6 Hz), 7.40 (d, 1H, J = 8 Hz), 6.21 (s, 1H), 5.38 (s, 2H), 3.28 (s, 3H). MS (ES) [m+H] calc'd for C13H11ClN3O2, 276.1; found 276.1.
References:
Takeda San Diego, Inc. EP1586571, 2005, A1 Location in patent:Page/Page column 43-44
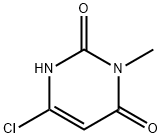
4318-56-3
608 suppliers
$9.00/5g
22115-41-9
461 suppliers
$5.00/5g
![2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile](/CAS/GIF/865758-96-9.gif)
865758-96-9
309 suppliers
inquiry
![2-[(6-CHLORO-2,4-DIOXO-3,4-DIHYDROPYRIMIDIN-1(2H)-YL)METHYL]BENZONITRILE](/CAS/GIF/865758-95-8.gif)
865758-95-8
124 suppliers
inquiry

74-88-4
351 suppliers
$15.00/10g
![2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile](/CAS/GIF/865758-96-9.gif)
865758-96-9
309 suppliers
inquiry
![2-[(6-CHLORO-2,4-DIOXO-3,4-DIHYDROPYRIMIDIN-1(2H)-YL)METHYL]BENZONITRILE](/CAS/GIF/865758-95-8.gif)
865758-95-8
124 suppliers
inquiry
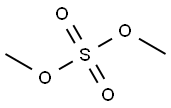
77-78-1
321 suppliers
$19.69/1ml
![2-[(6-Chloro-3,4-dihydro-3-Methyl-2,4-dioxo-1(2h)-pyriMidinyl)Methyl]benzonitrile](/CAS/GIF/865758-96-9.gif)
865758-96-9
309 suppliers
inquiry