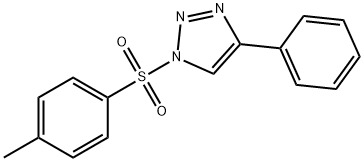
1H-1,2,3-Triazole, 1-[(4-methylphenyl)sulfonyl]-4-phenyl- synthesis
- Product Name:1H-1,2,3-Triazole, 1-[(4-methylphenyl)sulfonyl]-4-phenyl-
- CAS Number:884866-01-7
- Molecular formula:C15H13N3O2S
- Molecular Weight:299.35
Yield:884866-01-7 99%
Reaction Conditions:
with copper(I) thiophene-2-carboxylate;benzoic acid in benzene at 60; for 0.05 h;Flow reactor;
Steps:
Preparation of Triazole Compounds-1
Tosyl azide (0.30 g, 1.5 mmol) was dissolved in a benzene solvent (0.75 mL) in a dried 10 mL peach flask, and the dissolved tosyl azide was placed in a 10 mL SGE syringe and connected to a flow tube. after,It was mounted on a syringe pump. Then, ethynylbenzene (0.18 g, 1.8 mmol) and benzoic acid (0.092 g, 0.75 mmol) are completely dissolved in a benzene solvent (0.80 mL) in a dried 10 mL peach flask.CuTC (0.009 g, 0.045 mmol) was added to the solution in which benzoic acid was dissolved, and the mixture was dispersed in an ultrasonic disperser for 30 seconds.The solution in which the CuTC was dissolved was placed in a 10 mL SGE syringe, connected to a flow tube, and mounted on a syringe pump. In the syringe pump,The syringe type was 10 mL Scientific Glas Engineering with a syringe speed of 0.06 mL / min.to the next,After collecting the solution that proceeded the flow reaction at 60 ° Cin a 10 mL flask for 3 minutes,The solvent was concentrated at low pressure at 0 ° C.Then, flash silica gel column chromatography(Hexane: ethyl acetate = 10: 1)By using the following sulfonyl triazole compound (1) 4-phenyl-1-tosyl-1H-1,2,3-triazole represented as (4-phenyl-1-tosyl-1H-1,2,3-triazole)80 mg (99% yield) were obtained.
References:
KR2019/119509,2019,A Location in patent:Paragraph 0021; 0063-0067

98-59-9
625 suppliers
$9.00/5g
![1H-1,2,3-Triazole, 1-[(4-methylphenyl)sulfonyl]-4-phenyl-](/CAS/20200611/GIF/884866-01-7.gif)
884866-01-7
1 suppliers
inquiry
941-55-9
200 suppliers
$75.00/5G
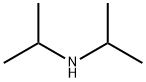
108-18-9
382 suppliers
$10.00/1g
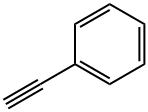
536-74-3
317 suppliers
$10.00/1g
![1H-1,2,3-Triazole, 1-[(4-methylphenyl)sulfonyl]-4-phenyl-](/CAS/20200611/GIF/884866-01-7.gif)
884866-01-7
1 suppliers
inquiry
![Benzeneethanimidamide, N,N-bis(1-methylethyl)-N'-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/847740-49-2.gif)
847740-49-2
0 suppliers
inquiry