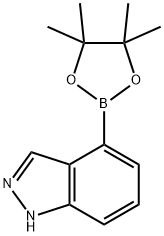
4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE synthesis
- Product Name:4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE
- CAS Number:885618-33-7
- Molecular formula:C13H17BN2O2
- Molecular Weight:244.1

73183-34-3
566 suppliers
$6.00/5g
186407-74-9
310 suppliers
$5.00/1g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
141 suppliers
$16.00/100mg
Yield: 60%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in dimethyl sulfoxide at 80; for 40 h;Product distribution / selectivity;
Steps:
5
To a solution of the 4-bromoindazole (500 mg, 2.54mmol) and bis(pinacolato)diboron (1.5 eq., 3.81mmol) in DMSO (2OmL) was added potassium acetate (3.0 eq., 7.61mmol, 747 mg; dried in drying pistol) and PdCl2(dppf)2 (3 mol%, 0.076mmol, 62 mg). The mixture was degassed with argon and heated at 800C for 40 h. The reaction mixture was allowed to cool and partitioned between water (5OmL) and ether (3 x 5OmL). The combined organic layers were washed with brine (5OmL), separated and dried (MgSO4). The crude material was purified by chromatography eluting with 30%-»40% EtO Ac-petrol to give an inseparable 3:1 mixture of the boronate ester (369 mg, 60%) and indazole (60 mg, 20%); this was isolated as a yellow gum which solidified upon standing to furnish (70) as an off- white solid. 1H NMR (400 MHz, J6-DMSO) (70) 1.41 (12H5 s), 7.40 (IH, dd5 J=8.4Hz, 6.9Hz)5 7.59 (IH, d, J=8.4Hz), 7.67 (IH5 d, J=6.9Hz), 10.00 (IH5 br s), 8.45 (IH5 s), and indazole: 7.40 (IH5 1), 7.18 (IH5 t, J=7.9Hz)5 7.50 (IH5 d5 J=9. IHz)5 7.77 (IH5 d5 J=7.9Hz)5 8.09 (IH5 s). Impurity at 1.25.
References:
LUDWIG INSTITUTE FOR CANCER RESEARCH;CANCER RESEARCH TECHNOLOGY LIMITED;INSTITUTE OF CANCER RESEARCH: ROYAL CANCER HOSPITAL;ASTELLAS PHARMA INC;PIRAMED LIMITED WO2007/42806, 2007, A1 Location in patent:Page/Page column 34-35

73183-34-3
566 suppliers
$6.00/5g

186407-74-9
310 suppliers
$5.00/1g
271-44-3
246 suppliers
$6.00/250mg
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
141 suppliers
$16.00/100mg
41748-71-4
144 suppliers
$19.00/250mg

73183-34-3
566 suppliers
$6.00/5g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
141 suppliers
$16.00/100mg
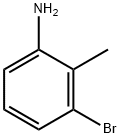
55289-36-6
280 suppliers
$5.00/1g
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
141 suppliers
$16.00/100mg

885698-70-4
36 suppliers
$115.00/500mg
![4-(4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-YL)-1H-INDAZOLE](/CAS/GIF/885618-33-7.gif)
885618-33-7
141 suppliers
$16.00/100mg