
4-fluoro-N-piperidin-4-ylbenzenesulfonamide hydrochloride synthesis
- Product Name:4-fluoro-N-piperidin-4-ylbenzenesulfonamide hydrochloride
- CAS Number:913634-50-1
- Molecular formula:C11H16ClFN2O2S
- Molecular Weight:294.7733
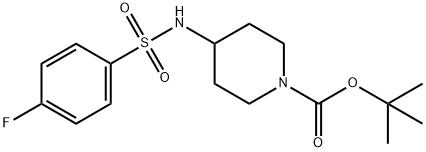
913634-49-8
16 suppliers
$235.00/250mg

913634-50-1
18 suppliers
$350.00/1g
Yield:913634-50-1 97%
Reaction Conditions:
with hydrogenchloride in ethyl acetate;
Steps:
III.2
Compound ?i-b (10 g, 27.899 mmol) was dissolved in ethyl acetate (120 mL) and saturated with gaseous HCl, and the reaction mixture stirred until all of the starting material was consumed. The mixture was concentrated to give amine hydrochloride salt as a white crystalline solid which was used in next step without further purification (8 g, 97%).[140] 1H-NMR(D2O): δ 7.61-7.68 (m, 2H), 7.10-7.17 (m, 2H), 3.34-3.44 (m, 2H),3.01-3.15 (m, IH), 2.93-3.09 (m, 2H), 2.04-2.15 (m, 2H), 1.63-1.75 (m, 2H).
References:
WO2006/115353,2006,A1 Location in patent:Page/Page column 15

79099-07-3
543 suppliers
$5.00/5g

913634-50-1
18 suppliers
$350.00/1g
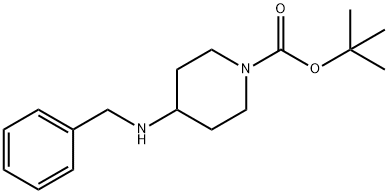
206273-87-2
80 suppliers
$45.00/50mg

913634-50-1
18 suppliers
$350.00/1g
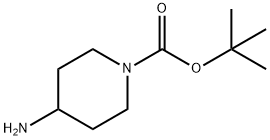
87120-72-7
20 suppliers
$6.00/1g

913634-50-1
18 suppliers
$350.00/1g