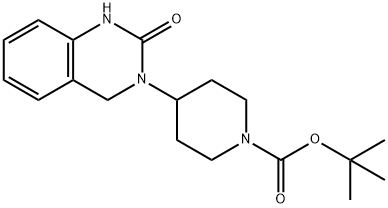
tert-butyl 4-(2-oxo-1,2-dihydroquinazolin-3(4H)-yl)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-oxo-1,2-dihydroquinazolin-3(4H)-yl)piperidine-1-carboxylate
- CAS Number:960221-97-0
- Molecular formula:C18H25N3O3
- Molecular Weight:331.41

79098-98-9
14 suppliers
inquiry
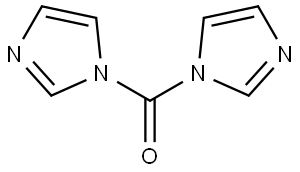
530-62-1
929 suppliers
$6.00/25g

960221-97-0
18 suppliers
$389.00/1g
Yield:960221-97-0 62%
Reaction Conditions:
with triethylamine in acetonitrile at 20; for 18 h;Reflux;
Steps:
3-(N-Boc-piperidin-4-yl)-3,4-dihydroquinazolin-2[1H]-one (5a) Preparation:
To a dry 500 mL round bottom flask was added 4a (11.5 g, 37.6 mmol) and acetonitrile (188 mL).Triethylamine (15.7 mL, 113 mmol) and 1,1’-carbonyl diimidazole* (7.40 g, 41.4 mmol) were added sequentially and the reaction solution stirred at room temperature for 1 h. An additionalportion of CDI (3.40 g, 0.5 equiv) was added and the flask fitted with a reflux condenser. The reaction was brought to reflux and maintained for 2 h. The solution was cooled to room temperature and stirred for 15 h. The acetonitrile was removed by rotary evaporation and theresulting residue dissolved in DCM. The white solid product 5a precipitated (5.97 g) and was isolated by vacuum filtration. The filtrate was washed once each with HCl (aq., 0.1 M) and sat.NaCl (aq.). The organic layer was dried (Na2SO4 anh.), filtered, and concentrated. The remaining residue was purified by automated flash column chromatography (30-100 % EtOAc:Hex) to yieldan additional 1.76 g of 5a.*We found the commercial 97% CDI reagent to be of ~ 90% purity by NMR.Physical State: white solid (7.74 g, 62 %), mp > 230 °C (with loss of CO2)IR: cast film (NaCl, DCM) 3215, 3127, 3063, 2976, 2859, 1681, 1604, 1478, 1366, 1299, 1217,1159, 1099, 909, 738 cm-11H NMR: (400 MHz, CDCl3) δ ppm 8.55 (s, 1 H) 7.16 (t, J = 7.1 Hz, 1 H) 7.02 (d, J = 7.3 Hz, 1H) 6.91 (t, J = 7.3 Hz, 1 H) 6.78 (d, J = 7.8 Hz, 1 H) 4.49 - 4.61 (m, 1 H) 4.31 (s, 2 H) 4.15 - 4.28(m, 2 H) 2.84 (br. s., 2 H) 1.60 - 1.80 (m, 4 H) 1.48 (s, 9 H)13C NMR: (100 MHz, CDCl3) δ ppm 154.9, 154.6, 137.0, 128.2, 125.4, 121.8, 117.7, 113.7,79.7, 51.3, 43.0, 28.5HR-MS (ESI): calc’d for C18H26N3O3 [M+H]+ 332.4235; found 332.19565
References:
Habay, Stephen A.;Miller, Julia M.;Bowler, Matthew M.;Manchak, Randi;Thomas, John Z. [Tetrahedron Letters,2018,vol. 59,# 36,p. 3389 - 3391] Location in patent:supporting information

79099-07-3
543 suppliers
$5.00/5g

960221-97-0
18 suppliers
$389.00/1g
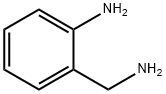
4403-69-4
104 suppliers
$9.00/1g

960221-97-0
18 suppliers
$389.00/1g
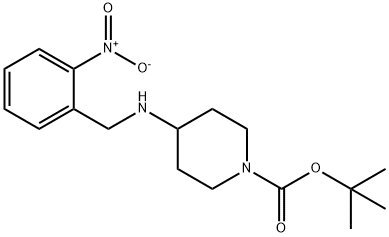
87120-79-4
19 suppliers
$309.00/1g

960221-97-0
18 suppliers
$389.00/1g
552-89-6
606 suppliers
$5.00/1g

960221-97-0
18 suppliers
$389.00/1g