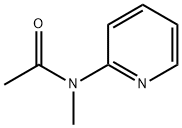
Acetamide, N-methyl-N-2-pyridinyl- synthesis
- Product Name:Acetamide, N-methyl-N-2-pyridinyl-
- CAS Number:61996-35-8
- Molecular formula:C8H10N2O
- Molecular Weight:150.18
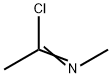
4597-87-9
168 suppliers
$17.00/1g

108-24-7
5 suppliers
$14.00/250ML

61996-35-8
9 suppliers
$285.00/250mg
Yield:61996-35-8 100%
Reaction Conditions:
at 70; for 4 h;
Steps:
4.1.8. N-Methyl-N-(2-pyridyl)acetamide (8)
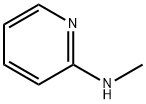
A mixture of 2-(methylamino)pyridine (857 mg, 7.92 mmol) and acetic anhydride (4.23 g, 41.4 mmol) was heated at 70 °C for 4 h. After removal of the solvent under reduced pressure, chromatography (AcOEt) of the residue gave 8 as a pale brown oil in quantitative yield. νmax (KBr) 1663, 1587, 1379 cm-1; 1H NMR (300 MHz, CD2C12) δ 8.44 (1H, ddd, J 0.8, 2.0, 4.8 Hz), 7.72 (1H, dd, J 2.0, 7.5 Hz), 7.29 (1H, d, J 8.1 Hz), 7.15 (1H, ddd, J 1.0, 4.8, 7.3 Hz), 3.31 (3H, s), 2.03 (3H, s); 13C NMR (75 MHz, CDC13, 55 °C) δ 170.7, 156.5, 148.9, 138.0, 121.5, 120.3, 35.4, 23.0; m/z (EI) 150; HRMS (EI): M+, found 150.0807. C8H10N2O requires 150.0794.
References:
Okamoto, Iwao;Terashima, Masayuki;Masu, Hyuma;Nabeta, Mayumi;Ono, Kaori;Morita, Nobuyoshi;Katagiri, Kosuke;Azumaya, Isao;Tamura, Osamu [Tetrahedron,2011,vol. 67,# 44,p. 8536 - 8543] Location in patent:experimental part
109-04-6
525 suppliers
$6.00/25g
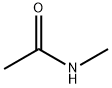
79-16-3
307 suppliers
$8.00/25g

61996-35-8
9 suppliers
$285.00/250mg
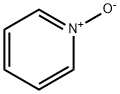
694-59-7
284 suppliers
$7.00/5g

79-16-3
307 suppliers
$8.00/25g

61996-35-8
9 suppliers
$285.00/250mg
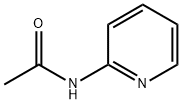
5231-96-9
121 suppliers
$60.00/50mg

74-88-4
341 suppliers
$15.00/10g

61996-35-8
9 suppliers
$285.00/250mg