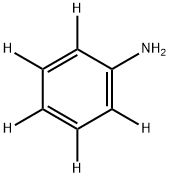
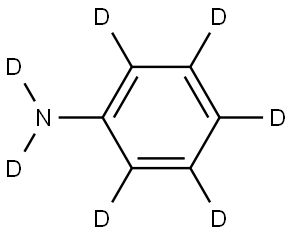
ANILINE D5 synthesis
- Product Name:ANILINE D5
- CAS Number:4165-61-1
- Molecular formula:C6H7N
- Molecular Weight:93.13
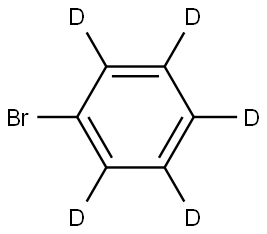
4165-57-5
174 suppliers
$56.29/2g

4165-61-1
120 suppliers
$56.00/1ml
Yield:4165-61-1 61.1%
Reaction Conditions:
Stage #1:bromobenzene-d5 with copper(l) iodide;potassium carbonate;4R-4-hydroxyproline in dimethyl sulfoxide
Stage #2: with ammonium hydroxide in dimethyl sulfoxide at 80;
Steps:
1.1-1 Synthesis Example 1-1) Synthesis of [Intermediate 1-a]
Bromobenzene-d5 (500 g, 3085 mmol), copper iodide (117.5 g, 615 mmol) in a round bottom flask, trans-4-hydroxy-L-proline (162 g, 1235 mmol), potassium carbonate (1279.5 g, 9255 mmol) and dimethyl sulfoxide 2500 mL were added and stirred.After the gas trap was installed, 2900 mL of ammonia water (28%) was slowly added dropwise. Stir at 80 °C overnight. After the reaction was completed, it was extracted several times with ethyl acetate and distilled water.After concentrating the organic layer under reduced pressure, column chromatography was performed with ethyl acetate and heptane to obtain 185 g (yield 61.1%) of liquid [Intermediate 1-1-a].[Intermediate 1-1-a] (185 g, 1887 mmol) synthesized above in a round bottom flask,Trichloroacetonitrile (326.6 g, 2257 mmol) and dichloromethane 1850 mL were added, and tin (IV) chloride (491.2 g, 1887 mmol) was added. After the flask was placed in an ice-bath, 1 M in dichloromethane (243.3 g, 2072 mmol) of boron trichloride was slowly added dropwise thereto, followed by reflux stirring for 24 hours.After completion of the reaction, the flask was placed in an ice-bath, and potassium carbonate (3126.5 g, 22616 mmol) was dissolved dropwise in methanol in a beaker.After dropping, the mixture was heated to remove dichloromethane and stirred under reflux for about 3 hours.After cooling to room temperature, the insoluble solid was filtered, the filtrate was concentrated under reduced pressure, dissolved in ethyl acetate, and washed with a 2-normal aqueous hydrochloric acid solution and water.The organic layer was treated with magnesium sulfate, concentrated under reduced pressure, and then chromatographed with ethyl acetate and hexane to obtain 41.6 g of [Intermediate 1-a]. (Yield 18.1%)
References:
SFC Ltd.;Lee Se-jin;Park Sang-u;Yang Byeong-seon;Kim Su-jin;Oh Hyeon-ju;Ryu Jeong-ho KR102081617, 2020, B1 Location in patent:Paragraph 0354-0359
4165-60-0
101 suppliers
$34.89/u

4165-61-1
120 suppliers
$56.00/1ml

62-53-3
675 suppliers
$10.00/1g

4165-61-1
120 suppliers
$56.00/1ml
1076-43-3
237 suppliers
$19.19/1pak

4165-61-1
120 suppliers
$56.00/1ml
14545-23-4
71 suppliers
$76.00/1ml

4165-61-1
120 suppliers
$56.00/1ml