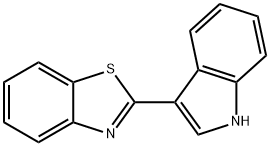
Benzothiazole, 2-(1H-indol-3-yl)- synthesis
- Product Name:Benzothiazole, 2-(1H-indol-3-yl)-
- CAS Number:31224-76-7
- Molecular formula:C15H10N2S
- Molecular Weight:250.32
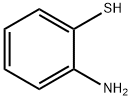
700-06-1
622 suppliers
$10.00/1g
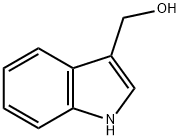
137-07-5
290 suppliers
$10.00/5g

31224-76-7
12 suppliers
$599.00/1g
Yield:31224-76-7 96%
Reaction Conditions:
with tetrabutylammonium bromide in N,N-dimethyl-formamide at 80; for 2.66667 h;
Steps:
2.6. General procedure for the synthesis of benzimidazoles or benzothiazoles in the presence of Pd(II)Cl2-BTP(at)MNPs catalyst
General procedure: A mixture of alcohol (1 mmol), 1,2-phenylenediamine or 2-aminothiophenol(1 mmol), Pd(II)Cl2-BTPMNPs (0.019 g, containing 0.09mol% Pd) and (1 mmol) tetrabutylammonium bromide (TBAB, 0.01 g)in DMF (5 mL) in a round-bottomed flask equipped with a condenser wasstirred at 80 °C. The progress of the reaction was monitored by TLC(eluent: n-Hexane/EtOAc, 4: 1 for benzimidazoles and n-Hexane/EtOAc, 6: 1 for benzothiazoles). The catalyst was separated by permanentmagnet and washed with EtOAc (10 mL). The crude product waspurified by recrystallization from EtOAc or EtOH to afford the purebenzimidazole. The benzothiazoles was obtained by recrystallizationfrom n-hexane/EtOAc (10: 1).
References:
Dehbanipour, Zahra;Zarnegareyan, Ali [Inorganic Chemistry Communications,2022,vol. 141,art. no. 109513]
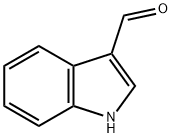
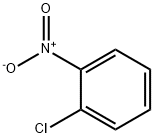
487-89-8
596 suppliers
$6.00/10g

137-07-5
290 suppliers
$10.00/5g

31224-76-7
12 suppliers
$599.00/1g
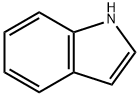
120-72-9
620 suppliers
$6.00/25g
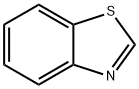
95-16-9
396 suppliers
$14.14/10gm:

31224-76-7
12 suppliers
$599.00/1g
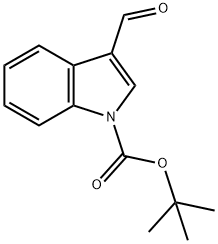
57476-50-3
116 suppliers
$6.00/1g

31224-76-7
12 suppliers
$599.00/1g