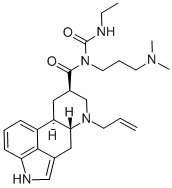
Cabergoline synthesis
- Product Name:Cabergoline
- CAS Number:81409-90-7
- Molecular formula:C26H37N5O2
- Molecular Weight:451.6
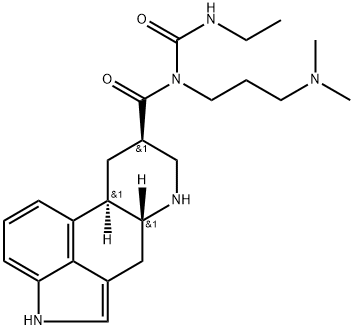
153415-36-2
12 suppliers
inquiry
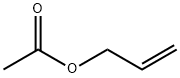
591-87-7
181 suppliers
$10.00/10g

81409-90-7
309 suppliers
$39.00/1mg
Yield:81409-90-7 92.3%
Reaction Conditions:
tetrakis(triphenylphosphine) palladium(0) in toluene at 20; for 2 h;
Steps:
5
EXAMPLE 5; Synthesis of 6-allyl-N-[3-(dimethylamino)propyl]-N-[(ethylamino)carbonyl]-ergoline-8?- carboxamide (Cabergoline).; To a suspension of 9.0 g (21.87 mmol) N- [3- (dimethylamino) propyl] -N- [(ethylamino)carbonyl)-ergoline-8?-carboxamide (XIX) in 250 ml of toluene 0.5 g of tetrakis (triphenylphosphine) palladium (0) and 5 ml of allyl acetate was added, and the reaction mixture was stirred at ambient temperature for 2 hours. The resulting mixture was washed with 100 ml of water. The organic layer was dried over anhydrous sodium sulphate. The dried solution was concentrated in vacuum and the product was purified on a silica plug to give 9.1 g (92.3%) of the title compound. 1H NMR (DMSO-d6, TMS, 500MHz) 8 1.10 (t, 3H, J=7. 2Hz, CONHCH2CH3) ; 1.47 (q, 1H, J=12. 4Hz, Hp-9) ; 1.62-1. 72 (m, 2H, CONCH2CH2CH2N (CH3) 2) ; 2.15 (s, 6H, CONCH2CH2CH2N (CH3) 2); 2.20-2. 30 (m, 2H, CONHCH2CH2CH2N (CH3) 2); 2.32-2. 40 (m, 2H, H-5, Hp-7) ; 2.54 (dd, 1H, J=14. 3Hz, 11.2Hz, Hα-4) ; 2.68-2. 84 (m, 2H, Ha-9, H-10); 3.08 (ddd, 1H, J=11. 3Hz, 3. 1Hz, 1. 8Hz, Ha-7) ; 3.14-3. 22 (m, 2H, CONHCH2CH3) ; 3.26 (dd, 1H, J=14. 7Hz, 7.3Hz, HX-N (6) CH2CH=CH2) ; 3.28-3. 38 (m, 2H, Hp-4, H-8) ; 3.48 (dd, 1H, J=14. 7Hz, 5.8Hz, Hy-N (6) CH2CH=CH2) ; 3.58-3. 68 (m, 2H, CONCH2CH2CH2N (CH3) 2); 5.15 (d, 1H, J=10. 3Hz, HX-N (6) CH2CH=CH2) ; 5.24 (d, 1H, J=17. 2Hz, Hy-N (6) CH2CH=CH2) ; 5. 88-5. 98 (m, 1H, N (6) CH2CH=CH2) ; 6.75 (d, 1H, J=7. 0Hz, H-12); 6.97 (s, 1H, H-2); 7.01 (t, 1H, J=7. 5Hz, H-13); 7.13 (d, 1H, J=8. 0Hz, H-14) ; 9.04 (t, 1H, J=5. 0Hz, CONHCH2CH3) ; 10.60 (s, 1H, N (1) H).
References:
WO2005/85243,2005,A2 Location in patent:Page/Page column 22
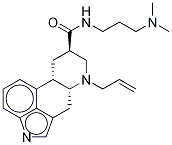
85329-86-8
37 suppliers
$420.00/1mg
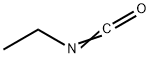
109-90-0
181 suppliers
$59.20/5g

81409-90-7
309 suppliers
$39.00/1mg
![Ergoline-8-carboxamide, N-[3-(dimethylamino)propyl]-1-[(1,1-dimethylethyl)dimethylsilyl]-N-[(ethylamino)carbonyl]-6-(2-propenyl)-, (8β)- (9CI)](/CAS/20210305/GIF/909724-71-6.gif)
909724-71-6
0 suppliers
inquiry

81409-90-7
309 suppliers
$39.00/1mg
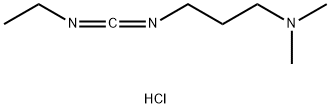
25952-53-8
783 suppliers
$9.00/25g
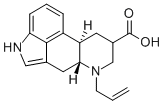
81409-74-7
42 suppliers
inquiry

81409-90-7
309 suppliers
$39.00/1mg
![(82)-N-[[[3-(Dimethylamino)propyl]amino]carbonyl]-N-ethyl-6-(2-propen-1-yl)-ergoline-8-carboxamide](/CAS/20211123/GIF/81409-91-8.gif)
81409-91-8
8 suppliers
inquiry
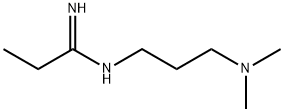
1892-57-5
302 suppliers
$5.00/1g

81409-74-7
42 suppliers
inquiry

81409-90-7
309 suppliers
$39.00/1mg