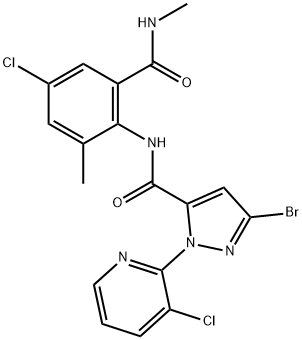
Chlorantraniliprole synthesis
- Product Name:Chlorantraniliprole
- CAS Number:500008-45-7
- Molecular formula:C18H14BrCl2N5O2
- Molecular Weight:483.15
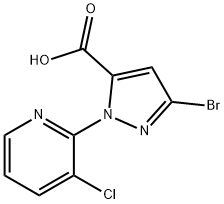
500011-86-9
168 suppliers
$8.00/250mg
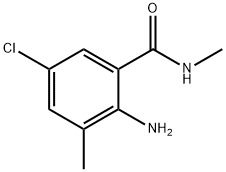
890707-28-5
159 suppliers
$5.00/100mg

500008-45-7
6 suppliers
$25.00/5mg
Yield:500008-45-7 97%
Reaction Conditions:
with 3-Methylpyridine;methanesulfonyl chloride in propiononitrile at -5 - 20; for 4 h;Product distribution / selectivity;
Steps:
13
EXAMPLE 13 Preparation of 3 -bromo-iV- [4-chloro-2-methyl-6- [(methylamino)carbonyl]phenyl] - l-(3-chloro-2-pyridinyl)-lH-pyrazole-5-carboxamide in propionitrileTo a mixture of 3-bromo-l-(3-chloro-2-pyridinyl)-lH-pyrazole-5-carboxylic acid (see PCT Patent Publication WO 03/015519 for preparation) (6.05 g, 20.0 mmol) and 2-amino-5-chloro-N,3-dimethylbenzamide (i.e. the product of Examples 1, 3, 4 and 5) (4.17 g,21.0 mmol) in propionitrile (18 mL) was added 3-picoline (5.06 mL, 4.84 g, 52 mmol). The mixture was cooled to -5 °C, and then methanesulfonyl chloride (1.86 mL, 2.75 g, 24 mmol) was added dropwise at -5 to 0 °C. The mixture was stirred for 1 h at 0 to 5 °C, and then for 3 h at room temperature. Then water (9 mL) was added dropwise and the mixture was stirred at room temperature for 1 h. The mixture was filtered, and the solids were washed with 3:1 propionitrile-water (2 x 4 mL), then with propionitrile (2 x 4 mL), and dried under nitrogen to afford the title compound as a nearly white powder, 9.37 g (97.0% uncorrected yield).
References:
E.I. DUPONT DE NEMOURS AND COMPANY WO2006/62978, 2006, A1 Location in patent:Page/Page column 28

500011-86-9
168 suppliers
$8.00/250mg

120374-68-7
7 suppliers
inquiry
74-89-5
7 suppliers
$13.44/25ML

500008-45-7
6 suppliers
$25.00/5mg

890707-28-5
159 suppliers
$5.00/100mg

943982-60-3
7 suppliers
inquiry

500008-45-7
6 suppliers
$25.00/5mg

4389-45-1
472 suppliers
$5.00/5g

500008-45-7
6 suppliers
$25.00/5mg
![4H-3,1-Benzoxazin-4-one, 2-[3-bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazol-5-yl]-6-chloro-8-methyl-](/CAS/20200611/GIF/500011-87-0.gif)