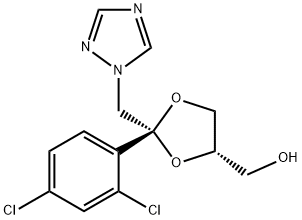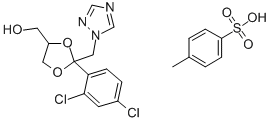
Cis -Tosylate synthesis
- Product Name:Cis -Tosylate
- CAS Number:154003-23-3
- Molecular formula:C20H19Cl2N3O5S
- Molecular Weight:484.35
Yield: 59.7%
Reaction Conditions:
with triethylamine in dichloromethane at -20 - 25; for 5 h;Time;
Steps:
6
9 g of compound IX-b (91%, 25 mmol, 1.0 eq) was added to a 500 ml reactor. 28.6 g of p-toluenesulfonyl chloride (150 mmol, 6 eq) and 70 ml of dichloromethane, cooled to -20 ° C, 15.2 g of triethylamine (150 mmol, 6 eq) was slowly added dropwise, and the temperature was kept below 25 ° C during the dropwise addition. After the dropwise addition was completed, the reaction was kept at 25 ° C for 5 hours. Add 70 ml of water to the reaction system. Stir for 30min, separate the liquid, collect the organic phase, and concentrate under reduced pressure to give the crude product 14g; The crude product was dissolved in 100 ml of ethyl acetate, and p-toluenesulfonic acid solution (5.5 g dissolved in 50 ml of ethyl acetate) was added dropwise at 25 ° C to precipitate a solid, and the cake was collected. Dry at 50 ° C to obtain crude p-toluenesulfonate. The crude product was recrystallized from 240 ml of acetonitrile, and the obtained refined product was dissolved in 60 ml of dichloromethane, then 50 ml of saturated sodium hydrogen carbonate solution was added, stirred and washed, and the organic phase was collected. It was concentrated to dryness under reduced pressure to give 7.3 g of Compound III-b.
References:
Jiangsu Aidi Pharmaceutical Co., Ltd.;Nanjing Ansailai Pharmaceutical Technology Co., Ltd.;Shen Xiaoning;Sun Jianhua;Liu Zhenxing;Chen Lulu CN104774195, 2018, B Location in patent:Paragraph 0069-0071; 0074-0075; 0082-0083
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

154003-23-3
47 suppliers
inquiry
58905-16-1
47 suppliers
inquiry

154003-23-3
47 suppliers
inquiry