
Dabigatran Etexilate iMpurity I synthesis
- Product Name:Dabigatran Etexilate iMpurity I
- CAS Number:255706-13-9
- Molecular formula:C14H21N3O2
- Molecular Weight:263.34
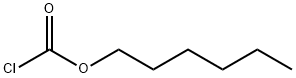
6092-54-2
288 suppliers
$6.00/1g
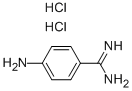
2498-50-2
387 suppliers
$6.00/250mg

255706-13-9
43 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydroxide in dichloromethane at 20; for 4 h;
Steps:
1.1 (1) Preparation of dabigatran intermediate condensate oily substance:
To the reaction flask, add 21.2 g (0.10 mol) of p-aminobenzamidine dihydrochloride (II), 150 ml of dichloromethane. Add 80 ml of 15% (0.12 mol) solution of sodium hydroxide at a controlled temperature of 20 deg.C. Add dropwise 16.5g (0.1 mol) n-hexyl chloroformate (III). React for 4h. The aqueous layer was separated and the dichloromethane was distilled off to obtain 26.4 g of an oily product of the condensate (I).
References:
Shandong Xinhua Pharmaceutical Co., Ltd.;Di, Jisheng;Zhao, Bin;Zheng, Zhonghui;Xu, Ling;Li, Chengpeng CN105732433, 2016, A Location in patent:Paragraph 0016; 0017