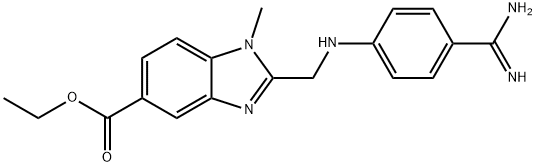
Dabigatran Impurity 9 synthesis
- Product Name:Dabigatran Impurity 9
- CAS Number:1408238-41-4
- Molecular formula:C19H21N5O2
- Molecular Weight:351.4
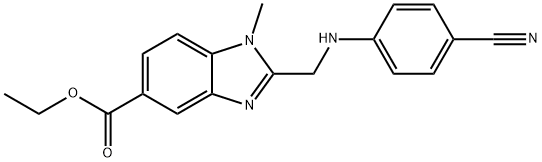
1117893-66-9
7 suppliers
inquiry

1408238-41-4
54 suppliers
inquiry
Yield: 89%
Reaction Conditions:
Stage #1:ethyl 2-(((4-cyanophenyl)amino)methyl)-1-methyl-1H-benzo[d]imidazole-5-carboxylate with hydrogenchloride in ethanol at 20; for 24 h;
Stage #2: with ammonium hydroxide in ethanol at 10 - 20;
Steps:
5.3 Step 3: Preparation of D-3
Intermediate D-2 (13.6 g, 41 mmol, 1.0 eq) and 200 mL of ethanol were added to a 500 mL reaction bottle, stirred and dissolved, and then 103 mL of 2N hydrochloric acid ethanol (205 mmol, 5.0 eq) was added, and the reaction was stirred at room temperature for 24 h. After the reaction is completed, the hydrochloric acid ethanol is distilled off, 300 mL of absolute ethanol is added to dissolve; cooled to 10 ° C., and 100 mL of ammonia water is added. After the addition, the reaction was stirred at room temperature overnight. The solvent was distilled off under reduced pressure, 200 mL of isopropyl ether was added to beat, filtered with suction, and dried to obtain 12.7 g of solid intermediate D-3 product, yield: 89%.
References:
Nantong Changyou Pharmaceutical Technology Co., Ltd.;Li Zebiao;Gu Wenchao;Zhang Qinghai;Ding Hongping;Lin Yanfeng CN110981858, 2020, A Location in patent:Paragraph 0060; 0062; 0067-0068
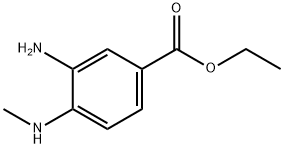
66315-23-9
82 suppliers
$33.00/500mg

1408238-41-4
54 suppliers
inquiry
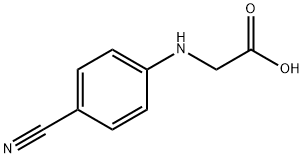
42288-26-6
555 suppliers
$14.00/1g

1408238-41-4
54 suppliers
inquiry